BrainXell
Background
BrainXell, Inc. specializes in the differentiation of human iPSCs to a variety of neuronal cell types that provide researchers biologically relevant systems for CNS drug discovery and toxicity screening. Researchers can choose from a variety of highly pure, functionally specialized subtypes that exhibit biochemical and functional characteristics of human neurons. These neurons provide an ideal in vitro experimental system for a wide range of neurophysiological investigations. Multi-electrode arrays (MEA) are used to evaluate neuronal activity of cell cultures and measure extracellular voltage changes that occur as neurons fire action potentials. Such measurements reveal firing patterns of individual neurons as well as patterns of neuronal networks that exist in the cell culture. The measurements are non-invasive and allow for repeated measurements over the lifetime of a cell culture. MEA can be used to study the physiological behavior of neurons, acute and long-term pharmacological responses, and disease phenotypes. This application protocol describes the culture of BrainXell Spinal Motor Neurons to perform consistent and reproducible MEA assays to investigate neuronal activity.
Contents
- One vial cryopreserved human neurons (options):
- Spinal Motor (BrainXell BX-0100-XX)
- Cortical Glutamatergic (BrainXell BX-0300-XX)
- Medium Spiny Neuron GABAergic (BX-0700-XX)
- BrainFast Motor #BX-2100, Glut #BX-2300, or GABA #BX-2400 (neuron specific) formerly known as Neuron Seeding Supplement
- BrainFast D4 (BrainXell #BX-2040) formerly known as Neuron Day 4 Supplement
- BrainFast SK (BrainXell #BX-2020) formerly known as Supplement K
Storage
- Immediately transfer the cryovial of neurons to a liquid or vapor nitrogen storage system.
- BrainFast supplements should be stored at -20°C (6 months) or -80°C (18 months). Return vial to -20°C between each time of use to maintain stability.
Additional Materials Needed
- DMEM/F-12 Medium, L-glutamine HEPES (Thermo Fisher Scientific #11330032)
- Neurobasal Medium (Thermo Fisher Scientific #21103049)
- BrainPhys Neuronal Medium (STEMCELL Technologies #05790)
- B-27 Supplement (Thermo Fisher Scientific #17504044)
- N-2 Supplement (Thermo Fisher Scientific #17502048)
- GlutaMAX Supplement (Thermo Fisher Scientific #35050061)
- Geltrex (Thermo Fisher Scientific #A1413201) or Cultrex (R&D Systems #3432-001-01)
- BDNF (Thermo Fisher Scientific #450-02)
- GDNF (Thermo Fisher Scientific #450-10)
- TGF-β1 (Thermo Fisher Scientific #100-21C)
- PDL-coated 24-Well CytoView MEA Plate (Axion Biosystems # M384-tMEA-24W)
- Refer to BrainXell Culture Plates PDL Coating Protocol v10.0
Equipment Needed
- Maestro Edge (Axion Biosystems)
- Alternate equipment is available for MEA/electrical assays. Protocols may need to be adjusted based on manufacturer recommendations.
Cell Culture Procedure
Day 0 Seeding Preparation
To seed (1) 24-well plate within the recommended seeding density you will need 1.3 – 3.3 million live cells. Review the Certificate of Analysis for each lot number to determine the expected total live cell count per vial.
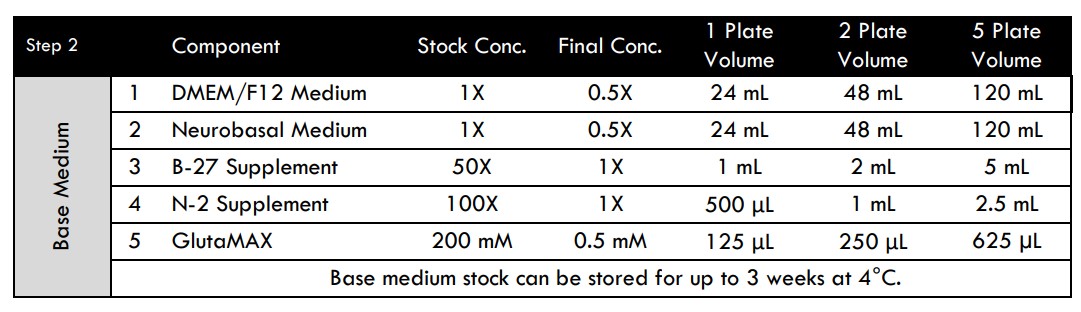
- Gather the Base and Seeding Medium components according to the recipe (see Media Compositions section below).
- Note that BDNF, GDNF, and TGF-β1 are lyophilized powders. Follow the manufacturer’s instructions for reconstitution and long-term storage. We recommend creating stock solutions at the listed concentrations shown in the Media Compositions section below.
- Working in a cell culture hood (biological safety cabinet), combine all Base Medium components in an appropriately sized sterile container to create a working stock.
- Prepare a pre-diluted Cultrex solution: add 360 μL of cold DMEM/F12 into a 40 μL aliquot of frozen Cultrex taken directly from the -80 freezer. Mix to dissolve and store at 4°C until it is time to add to medium during Step 21.
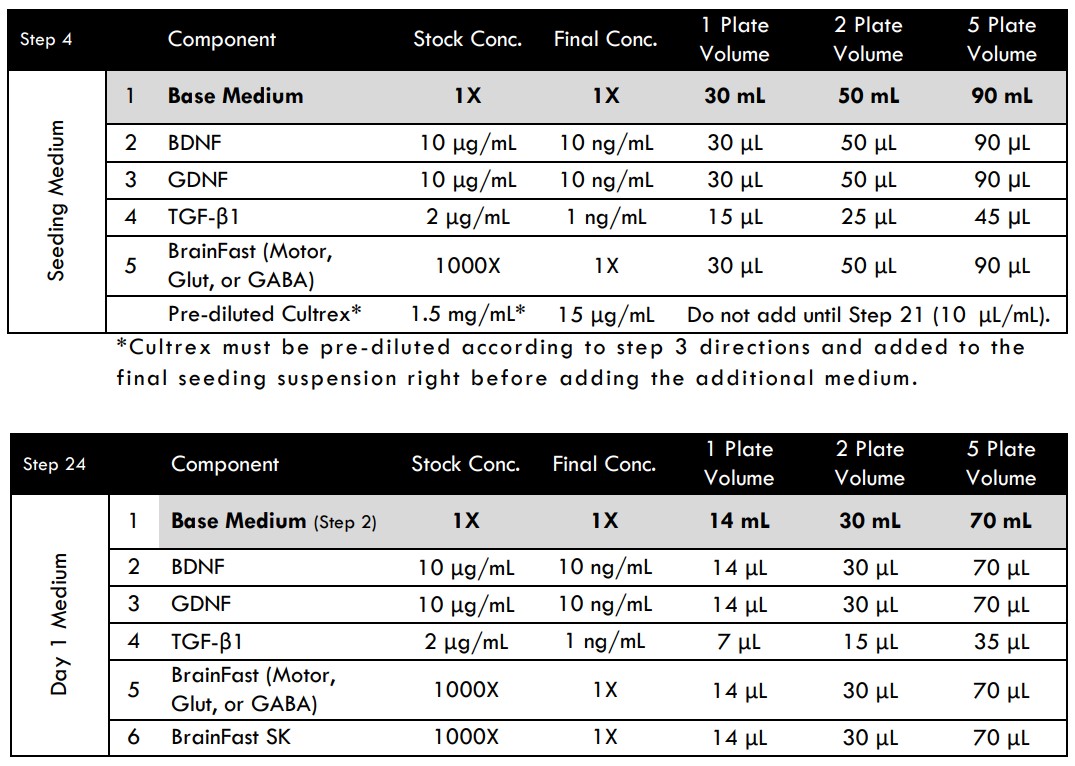
- Prepare Seeding Medium without Cultrex by adding BrainFast Motor supplement, BDNF, GDNF, and TGF-β1 to the Base Medium. Note: Cultrex will be added at a later step
- Allow the Seeding Medium to equilibrate to room temperature for at least 15 minutes prior to use. Do not warm the medium in a 37°C water bath. Culture plates should also be at room temperature prior to use.
- Prepare one 50-mL conical tube: add 3 mL of the Seeding Medium lacking Cultrex.
- Prepare one microcentrifuge tube: add 25 μL of Trypan Blue solution for cell counting.
Day 0 Seeding the Neurons
- Remove the cryovial of cells from nitrogen storage and place in a 37°C water bath. To minimize contamination, avoid submerging the cap. Gently move the vial within the bath to increase the rate of thawing.
- As soon as the last of the ice melts, which will take ~75-90 seconds, remove the cryovial from the water bath. Disinfect the vial by spraying it with 70% ethanol before transferring it into the cell culture hood.
- Using a P1000 pipette, add 500 μL Seeding Medium to the cryovial at a rate of ~2-3 drops/sec. This process should take about 10 seconds.
- Gently transfer all contents from the cryovial (~1 mL) to the 50-mL prepared 50-mL conical tube.
- To collect any residual cells, add an additional 1 mL of Seeding Medium to the cryovial and then transfer to the 50-mL tube. There should now be 5 mL total suspension in the tube.
- Gently swirl the conical tube and pipette up and down 3-5 times to ensure cells are evenly suspended in medium. Transfer 25 μL of cell suspension to the microcentrifuge tube prefilled with 25 μL Trypan Blue solution and pipette up and down a few times to mix.
- Centrifuge the diluted cells in the 50-mL tube at 1115 rpm (200xg) for 5 mins.
- While the cells are centrifuging, count the cells using the prepared Trypan Blue/cell suspension.
- Count the number of viable and dead cells using a hemocytometer to determine the live cell concentration (live cells/mL) and viability.
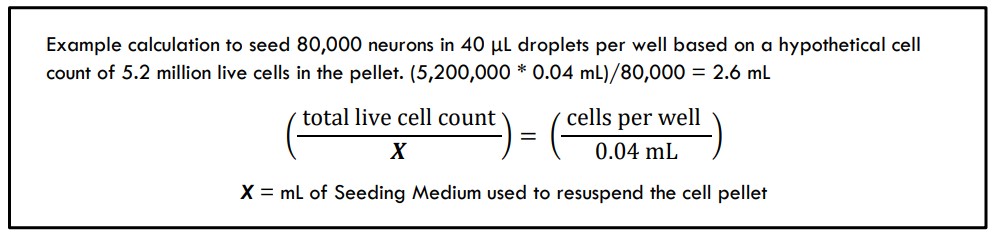
- Based on your cell count, calculate the total live number of cells that are in the 5 mL of suspension.
- Ex: cell count concentration was determined to be 1.04 million live cells/mL * 5 mL suspension = 5.2 million live cells.
- Calculate the volume of Seeding Medium you will use to resuspend the pellet to reach your target seeding density.
- Calculate the volume of Seeding Medium you will use to resuspend the pellets to create 2X concentrations of each cell type. The individual suspensions will be combined to make a final combined 1X cell suspension at the desired ratio.
- A recommended seeding density is 50,000 – 120,000 viable neurons/40 μL addition per well.
- After centrifugation, carefully remove the medium without disturbing the cell pellet.
- Immediately add Seeding Medium (that does not contain Cultrex) to the cell pellet to reach the calculated volume of your seeding suspension. Resuspend the cells by gently pipetting until the suspension appears homogeneous and no clusters are visible.
- Seed a 40 μL droplet (dot) of cells over the electrodes in each well in the MEA plate. Complete one well at a time being careful not to touch the electrodes with the pipette tip to prevent scratching of the surface.
- In a new sterile tube: add together the appropriate volumes of the 2X neuron and 2X astrocyte suspensions to create a final 1X seeding suspension. Gently mix the neurons and astrocytes together using a P1000 pipette to create a homogeneous mixed suspension.
- Seed a 40 μL droplet (dot) of cells over the electrodes in each well of the MEA plate. Complete one well at a time being careful not to touch the electrodes with the pipette tip to prevent scratching of the surface.
- Transfer the plate to a humidified incubator at 37°C with 5% CO2 for 30-60 minutes.
- At 30 minutes, check the cells under a microscope to make sure they have adhered to the surface. If they appear to be loosely attached, wait an additional 30 minutes before proceeding with the next step to prevent dislodging the cells from the electrodes upon addition of medium.
- Add pre-diluted cold Cultrex to the remainder of the Seeding Medium. You will need to add 10 µL of diluted Cultrex per each 1 mL of Seeding Medium.
- Example for (1) plate: add 150 µL of pre-diluted cold Cultrex to 15 mL of room-temp Seeding Medium; mix well.
- Gently add 460 μL of the Seeding Medium containing Cultrex to each well. Aim away from the center to prevent direct washing of medium over the cells.
- Transfer the plate back to the humidified incubator at 37°C with 5% CO2. The day of cell plating (today) is designated as Day 0.
*Note: The entire thawing and initial seeding process should be completed within 1.5 hours. Post-thaw viability and overall cell health could be severely impacted and lead to an unsuccessful culture if the process is too long.
Day 1 Medium Replacement
- On Day 1 (24 hours after seeding), prepare fresh Day 1 Medium.
- Gently remove 460 μL media from each well, leaving 40 μL/well.
- Gently add 460 μL of Day 1 Medium to the entire plate for a total volume of 500 μL/well.
Day 4 Medium Addition
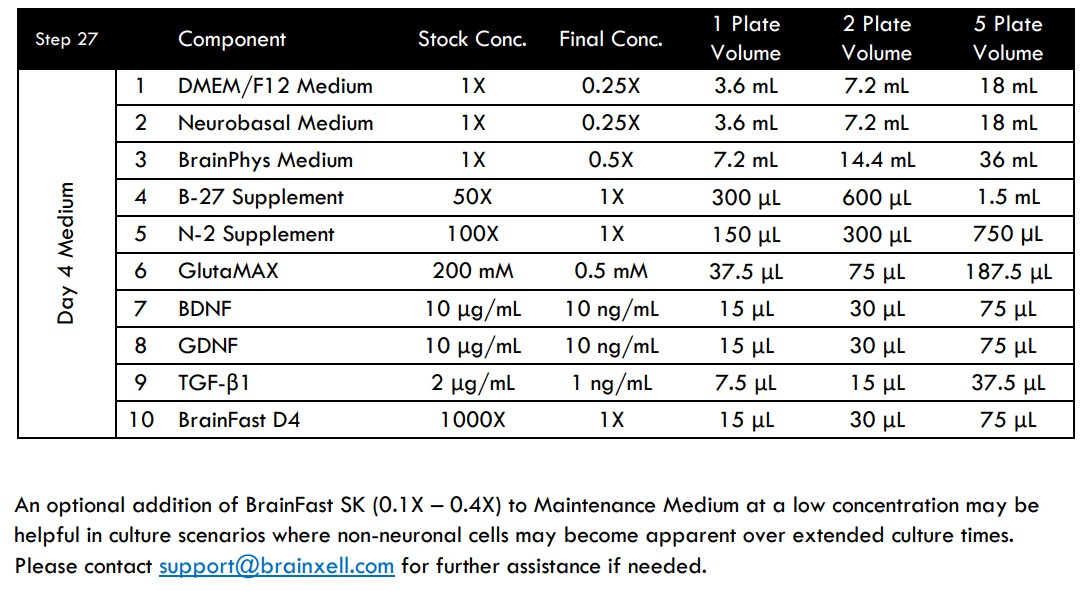
- On Day 4 (96 hours after seeding), prepare fresh Day 4 Medium.
- Gently add 500 μL/well of Day 4 Medium to the entire plate for a total volume of 1 mL/well.
Day 7 and Onward Medium Changes
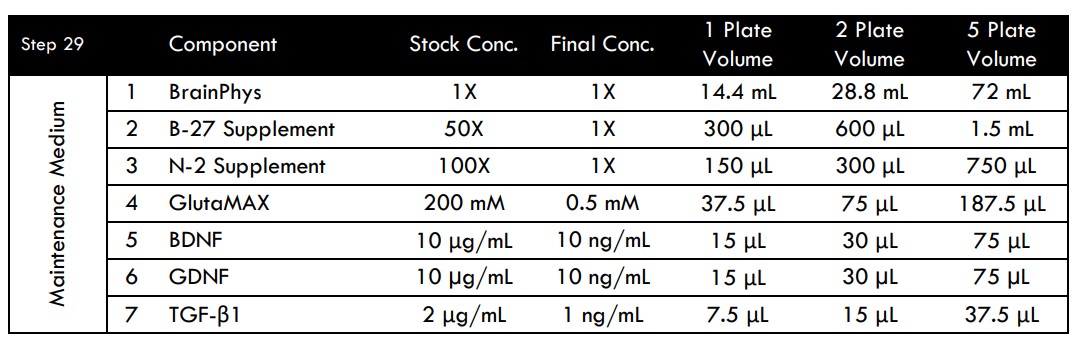
- Change half the medium (500 μL/well) twice weekly (Day 7, 11, 14, 18, etc.) using Maintenance Medium freshly prepared on each day of use.
- Note: Higher cell densities may need media changes more often. If the media is changing color (yellowing) sooner than 3 days, complete an early medium change.
MEA Assay
Neural activity should be present to some degree by 12 days in vitro and will reach a plateau around 21 days in vitro.
- For each recording, transfer the plate from the incubator to a Maestro Edge pre-equilibrated to 37℃ and 5% CO2.
- Place the MEA plate into the instrument and allow an additional 10-15 minutes before starting the acquisition to let the system and culture equilibrate.
- Record for 10 minutes or your desired length of time.
- Return the plate to the incubator to maintain the culture between recordings.
Note: Any required medium changes that fall on a recording day should be completed after recordings are acquired to minimize random variation from reading to reading.
Specific seeding density for MEA activity
If attempting to achieve electrical activity as quickly as possible for your experiments, it is recommended to seed at a higher density of 90,000-100,000 neurons/well.
Alternately, if attempting to perform a long-term study (75+ days in culture) it is recommended to seed at a lower density of ~45,000 neurons/well. Starting in the second week of culture, Maintenance Medium can be supplemented with 0.2X BrainFast SK (i.e. 4 μL of BrainFast SK into 20 mL of medium) if proliferation of flat cells is a concern. Perimeter wells on the plate should be monitored over time for reduced volumes due to heightened evaporative effects near the plate edges. If some wells begin to show less volume over time, the lower well volumes can be increased by adding additional fresh Maintenance Medium to the wells at the time of the half medium change.
MEA Media Compositions for Spinal Motor, Glutamatergic, or Medium Spiny GABAergic Neurons