Chvatal SA, Loghun MT, Hayes HB, Millard DC, Karumbaiah L.
American Association for Cancer Research, 2020
Immune-cell mediated cytotoxicity
Maestro Z: dynamic cell tracking
Dynamic Cytotoxicity Assay
Impedance Assay Measures Diverse Cell Properties
The need for simple, reliable, and predictive in vitro assays is highlighted by growing applications in cell manufacturing and immuno-oncology. The Maestro Z platform simplifies in vitro cell-based assays with an easy to-use benchtop system. The Maestro Z uses impedance technology to characterize cells cultured directly onto a planar electrode embedded in the substrate of each well on the plate, with sensitivity to each of the following four endpoints for in vitro assessment:
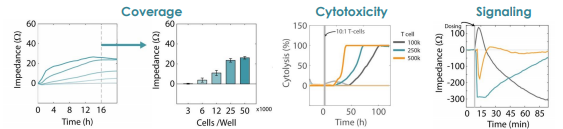
- Coverage – the change in impedance is directly related to the number of cells covering the electrode.
- Cytotoxicity – dynamic monitoring of cell viability compared to untreated controls facilitates measurements of the degree and speed of cytotoxic activity.
- Morphology – the size, shape, and intercellular tight junctions of adherent cells significantly impact the measured impedance signal.
- Signaling – small changes in cell shape or cytoskeleton organization are detected in response to intracellular signaling events
Impedance Assay is Directly Correlated with Cell Viability
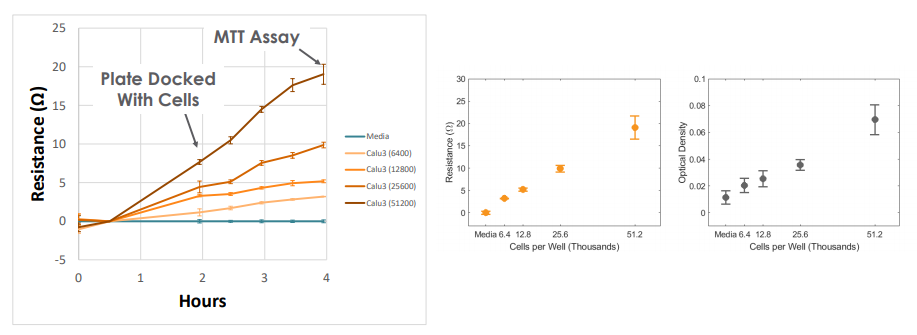
To validate impedance-based monitoring of cell viability, Calu-3 cells were added to a CytoView Z plate with varying number of cells per well and monitored for four hours on the Maestro Z platform. The change in resistance was correlated with the number of cells seeded upon docking into the instrument, and the resistance continued to increase as the cells adhered and flattened on the surface. At four hours post-seeding, the plate was removed and an MTT assay was performed in the CytoView Z plate. The resistance measured with the Maestro Z platform was linear with respect to cell number and directly correlated to the MTT assay readings from the same wells.
Continuous Monitoring of Cell Viability to Quantify Cytotoxicity
free measurement with the Maestro Z can be used to track cytotoxicity over time. Here, A549 cells were seeded in the CytoView Z plate, allowed to attached, spread, and proliferate, and then dosed at 24 hours post-seeding. Tergazyme, the full lysis control, immediately killed the cells and returned the impedance to baseline values. The no treatment control and vehicle control approached 100% confluence and remained unchanged over the 48 hours post-dose. The doxorubicin had a dose-dependent effect, with only the 1 µM concentration killing the cells, as evidenced by the progressive reduction in impedance over the post-dose time period.
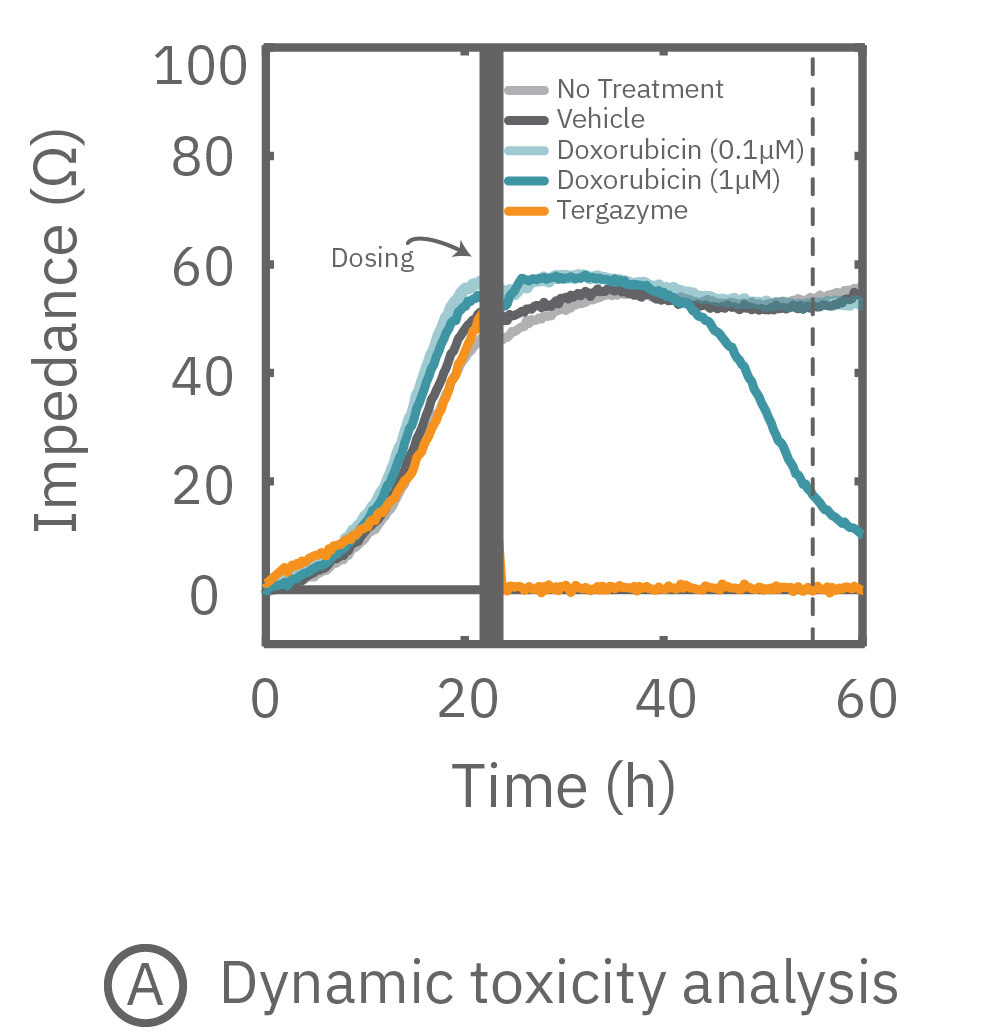
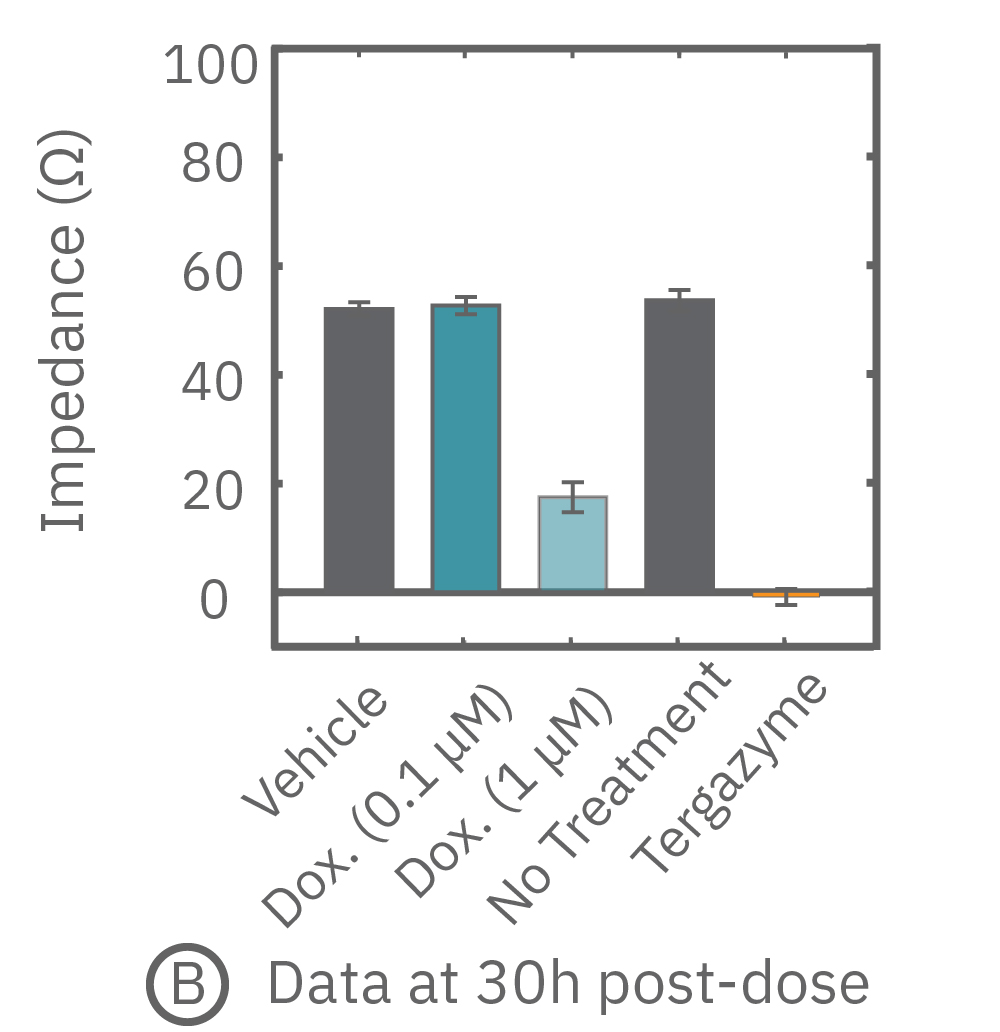
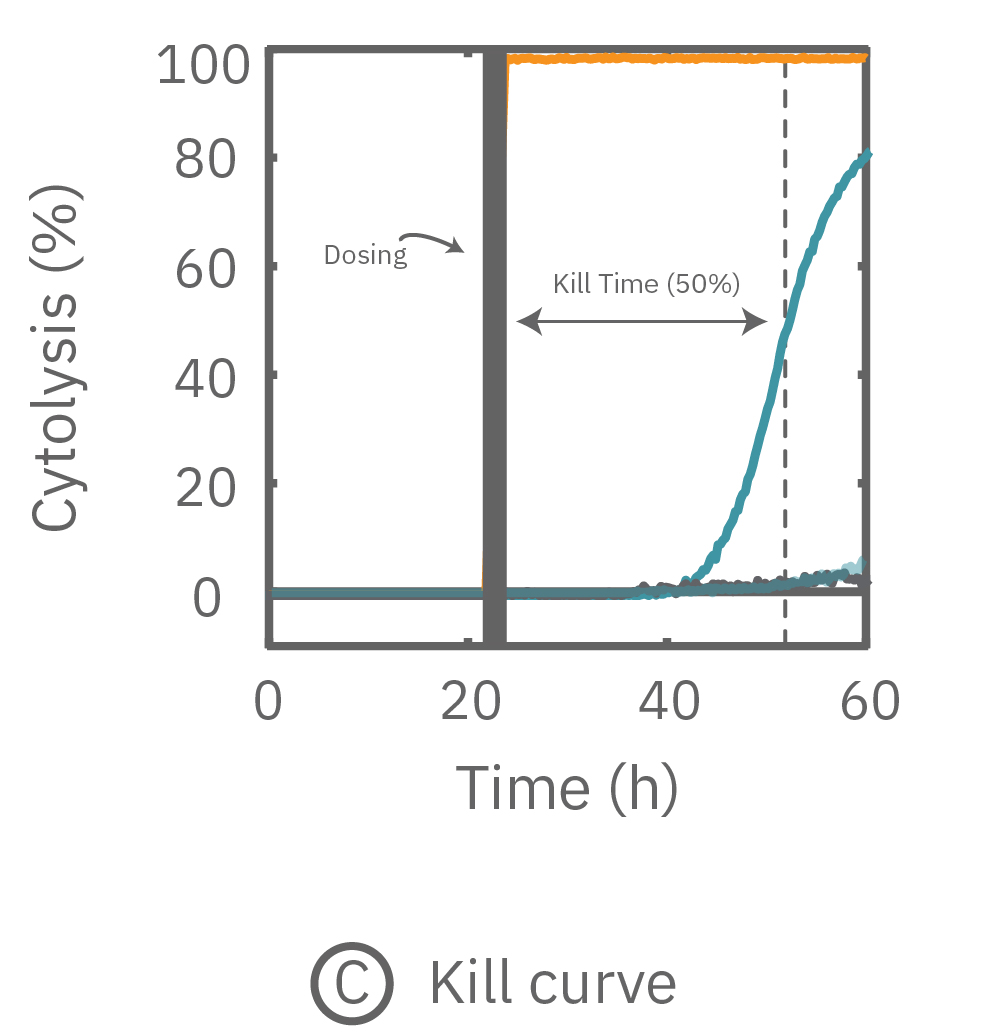
Percent cytolysis was computed by comparing treated wells to the “no treatment control”, with 0% cytolysis indicating similar measurements from the treated and untreated wells and 100% cytolysis marking a return to baseline impedance values. The doxorubicin (1 µM) treatment showed progressive cytolysis over the postdose time window, with 50% of the cells dying 20 hours post-dose (KT50).
Immune Cell-Mediated Cytotoxicity
Dynamic Tracking of T Cell-mediated Killing of U87 Glioblastoma
Impedance was tracked continuously for U87 cells plated at three different densities (10k, 25k, and 50k cells per well) using the Maestro Z system (left). The increase in impedance correlated to the density of the cells, with higher cell densities producing a greater change in impedance. The addition of activated human T cells resulted in a decrease in the impedance signal (right), consistent with T cell-mediated lysis of U87 cells, while untreated wells showed a continual increase in impedance, consistent with continued cell proliferation.
Concentration-dependent T Cell-mediated Kill Time
Treated wells were compared to untreated controls to compute T cell-mediated cytolysis. T cells were added in increasing number, but maintaining the same ratio of effector cells to target cells (10:1). Overall, higher T cell densities led to a faster killing of U87 cells (left). The time required to kill 50% of the U87 cells (KT50) was approximately 35 hours in the wells with 500k T cells, as compared to approximately 80 hours in the wells with 100k T cells.
Maestro Z: Dynamic Cell Tracking
Impedance Technology
The flexibility and accessibility of cell-based models has allowed complex human biology to be reproduced in vitro for applications in toxicology, disease-in-a-dish modeling, and drug/therapy discovery.
Glioblastoma (GBM) is an aggressive form of brain cancer that has no effective treatments and a prognosis of only 12-15 months. Immune system effector T cells hold promise for future cancer therapy due to their high specificity and innate cytotoxicity. Assessing the effectiveness and potency of T cell therapies benefits from dynamic cell tracking.
Axion BioSystems’ Maestro Z platform offers impedance-based cell analysis for real-time, label-free monitoring of cells. Here, we present a characterization of immune-cell mediated killing of glioblastoma cells in vitro using the Maestro Z.
The impedance is measured from electrodes embedded in the bottom of each well. As cells cover more of the electrode, the impedance increases. Similarly, if a perturbation kills the attached cells, the impedance will decrease.
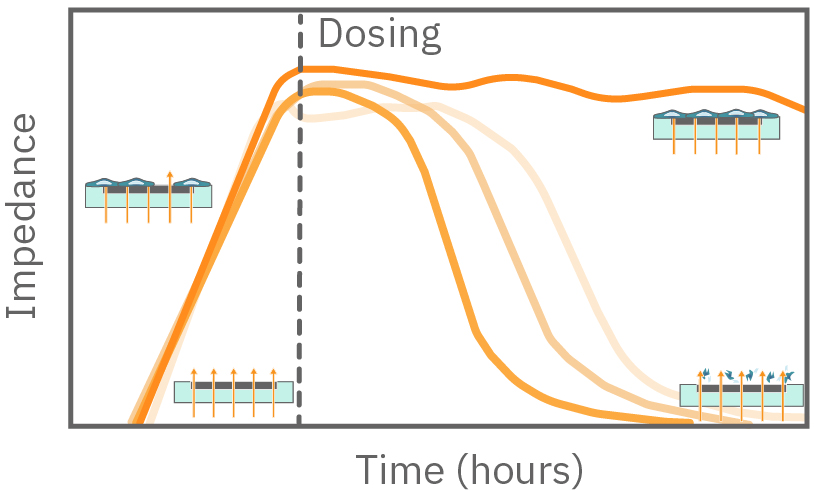
Impedance-based cell analysis provides measurements related to the attachment, proliferation, and coverage of attached cells, as well as the dynamics of cytotoxicity or changes in cell conformation and morphology as a result of cell signaling.
The Maestro Z Platform
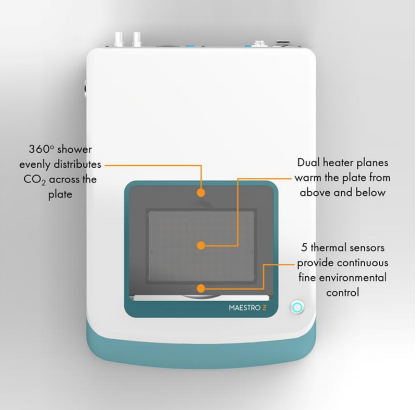
- Label-free, non-invasive tracking of cultured cells
- Integrated environmental control provides a stable environment for short- and long-term studies with a small benchtop footprint
- Automatic and continuous cell monitoring from 96 wells simultaneously
- “One button setup” automatically docks the plate and adjusts temperature and CO2 levels
- Powerful data analysis to focus on the science, while AxIS Z handles the details with simple setup and automatic experiment tracking
- See your cells with the viewing window included in each well of the CytoView Z 96-well plate
- State-of-the-art electrode processing chip (BioCore v4) enables advanced endpoints, such as barrier index, to further characterize cell-based models
Experimental Methods

(A) U87MG, hereafter referred to as U87 cells, glioblastoma cells (Cat. HTB-14) were obtained from ATCC. T cells were obtained from a patient (line H24FW). CytoView Z plates were coated with 100 µL PDL and incubated overnight. After 24 hours, 100uL of U87 medium was added for a baseline measurement. The U87 cells were then added at 10k, 25k, and 50k cells per 100 µL to 12 wells each. (B) The impedance measurements were then acquired continuously for the duration of the experiment with the Maestro Z platform. T cells were activated 24 hours before addition to the designated wells. (C) All analyses were performed with the AxIS Z software.
Conclusions
-
The Maestro Z platform enables dynamic, label-free cell tracking using impedance technology, providing measurements related to the attachment, proliferation, and coverage of attached cells, as well as the dynamics of cytotoxicity or changes in cell conformation/morphology following perturbations.
-
The impedance assay increased linearly with the number of cells seeded per well, and was directly correlated with the MTT cell viability assay.
-
T cell-mediated killing of the U87 gliobastoma cell line was easily detected as a decrease in impedance over time after administration of the T cells to the target wells, as measured relative to untreated controls.
-
The dynamic cell tracking allowed quantification of the T cell-mediated kill time for assessment of potency. In this study, the time to kill 50% of the target cell population was shorter with increasing T cell population, even with the effector-to-target ratio kept constant.
-
This proof-of-concept study demonstrates that dynamic cell tracking via an impedance-based assay can be used to assess T cell-mediated killing of gliobastoma cells. Future work will include patient derived glioblastoma and T cells, as well as chimeric antigen receptor T cell modifications.