Sections included in this whitepaper:
Why measure cardiac activity?
In vitro models are a proven powerful strategy for studying disorders of the human heart. Many of these disorders are the result of subtle changes to cardiomyocyte excitability, contractility, or both. The Maestro platform captures live cell activity from cardiomyocytes in real-time, providing you the functional cell information you have been missing.
What is MEA?
Axion’s microelectrode array (MEA) plates have a grid of tightly spaced electrodes embedded in the culture surface of each well. Electrically active cells, such as cardiomyocytes, can be cultured over the electrodes. Over time, as the cultures become established, cardiomyocytes can form a beating syncytium. The Maestro makes it easy to record spontaneous or evoked electrical activity from each electrode on a microsecond timescale, providing both temporally and spatially precise data.
The complete cardiac assay
The Maestro MEA platform is the next generation of electrophysiology plate readers. Record the four key measures of functional cardiac performance, label-free and in real time in every well of the multiwell plate using a single system.




Action Potential >>
How does it work?
The theory behind LEAP is similar to that of the patch clamp technique, where the recorded signal amplitude is proportional to the sealing resistance between the electrode and the cell. The LEAP induction process increases the coupling between the cells and electrode, which enables recording of an action potential signal rather than a field potential signal.
What can you measure?
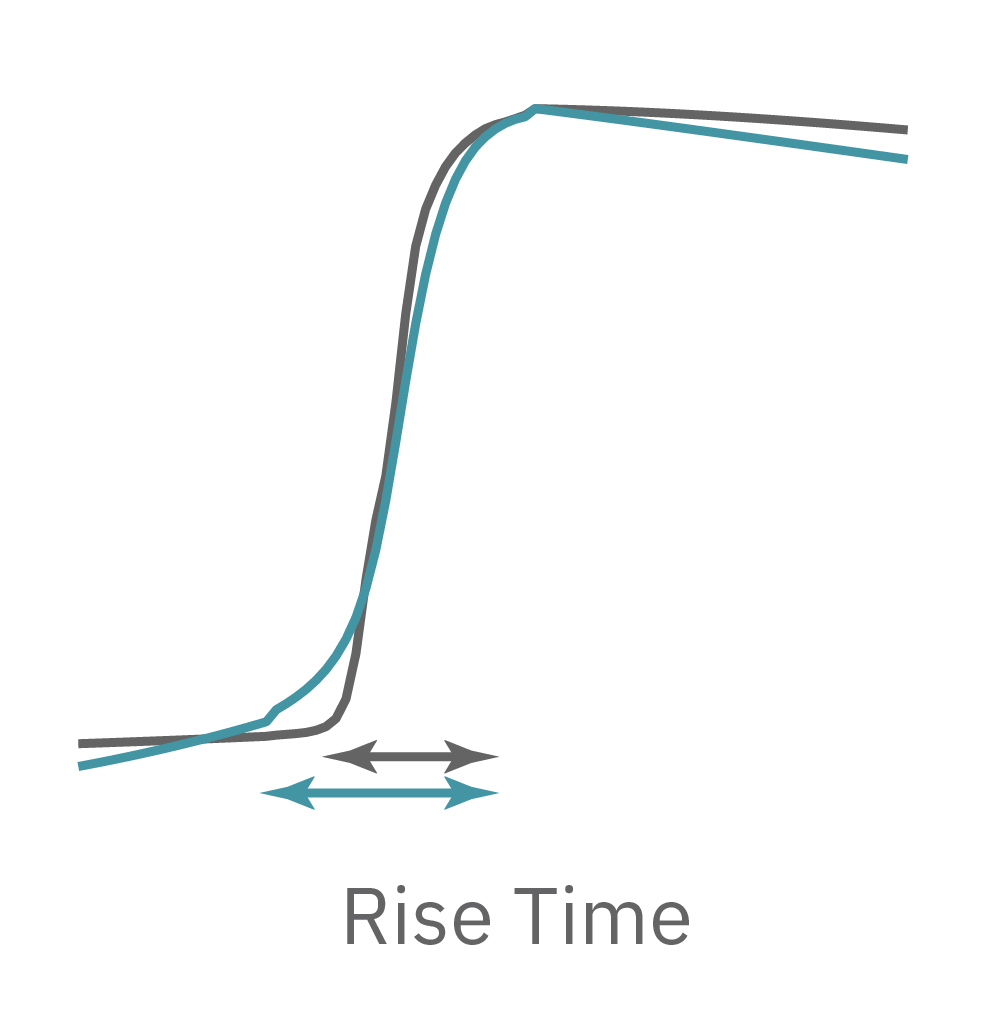
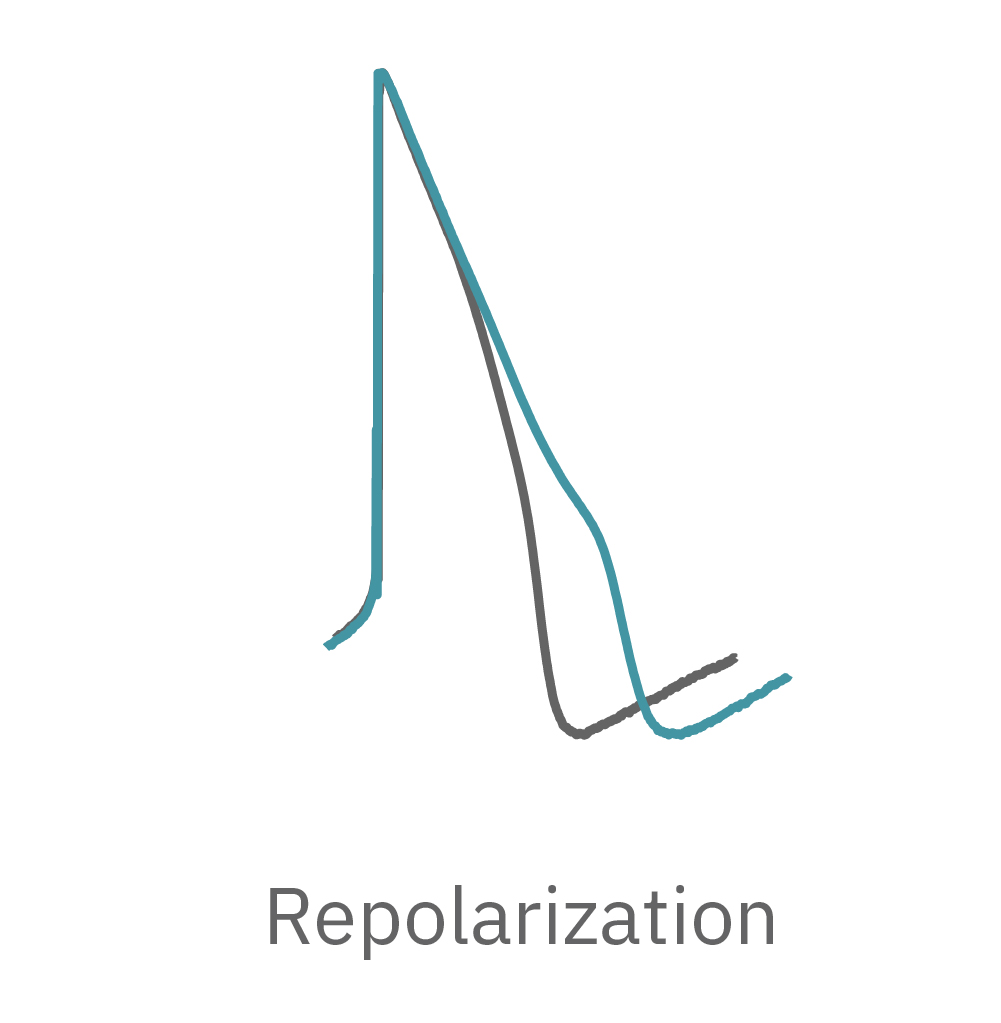
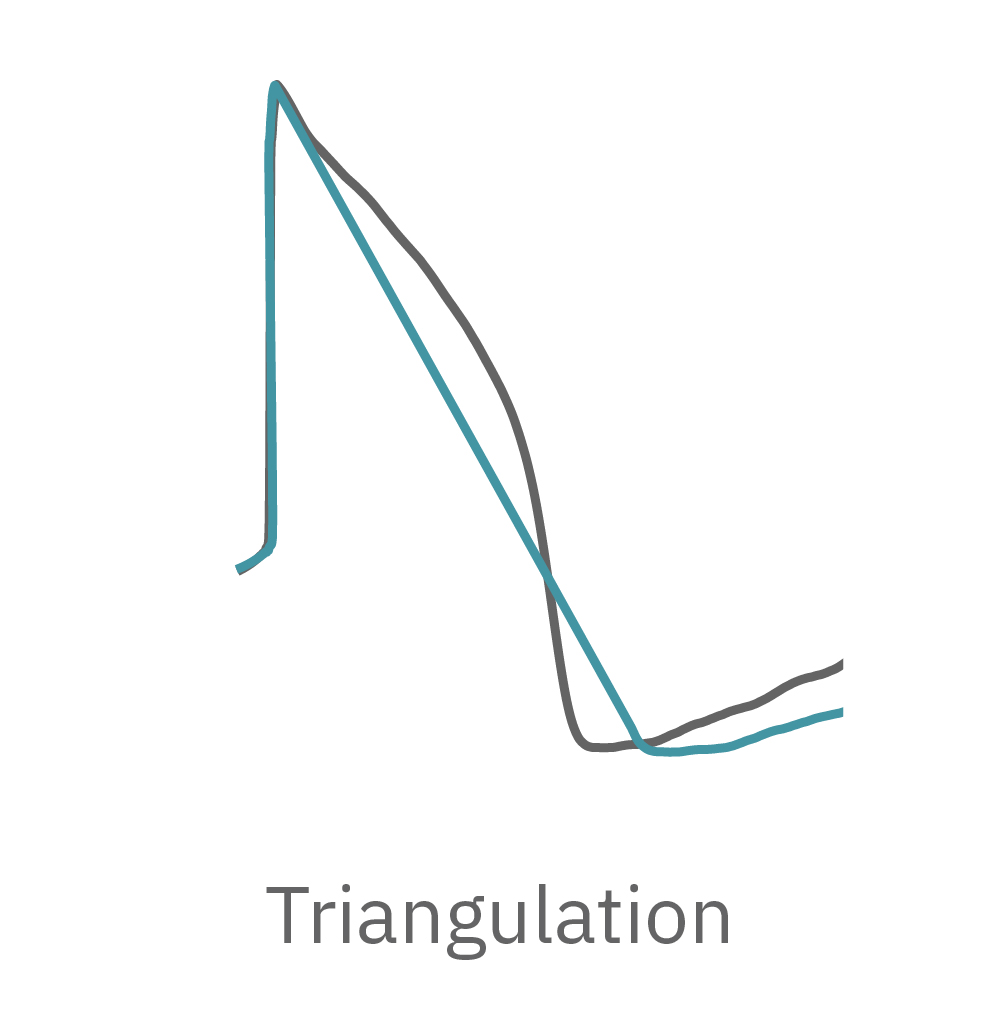
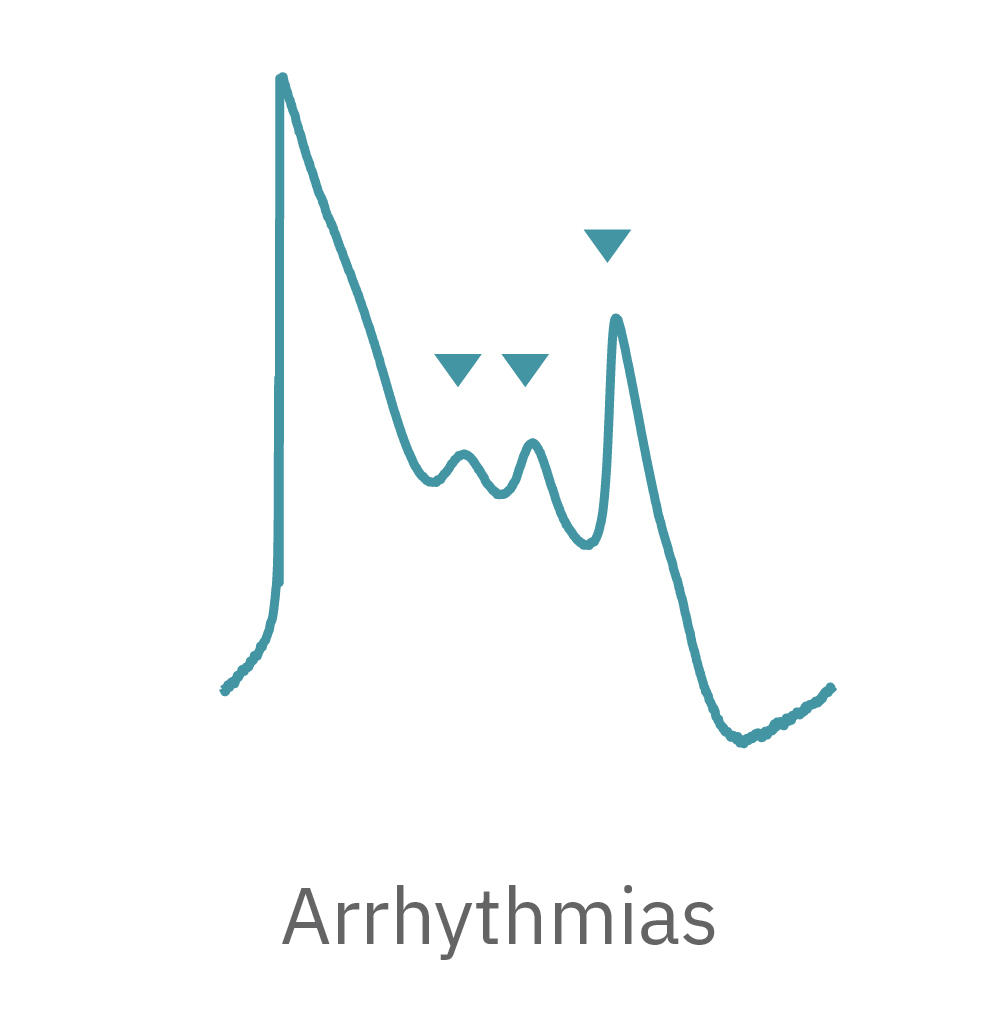
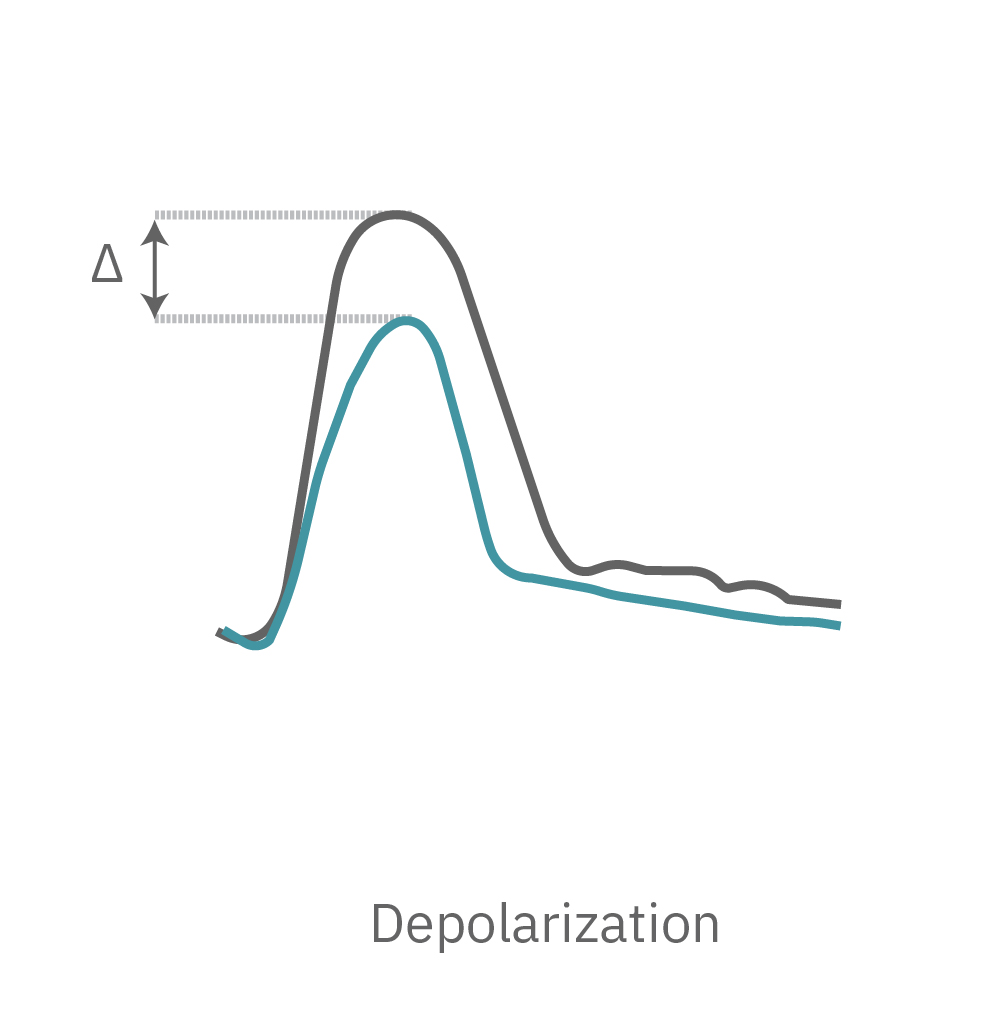
The Maestro MEA system detects key parameters of the cardiomyocyte action potential waveform, including depolarization (rise time), repolarization (APD30, APD50, APD90), triangulation, and irregular beating (arrhythmia).
LEAP case studies in pharmacology
LEAP was benchmarked against the cardiac field potential assay, establishing reliability and accuracy in tracking the cardiac action potential. Detection of hiPSC-CM action potential triangulation with LEAP is consistent with previous reports using manual patch clamp.
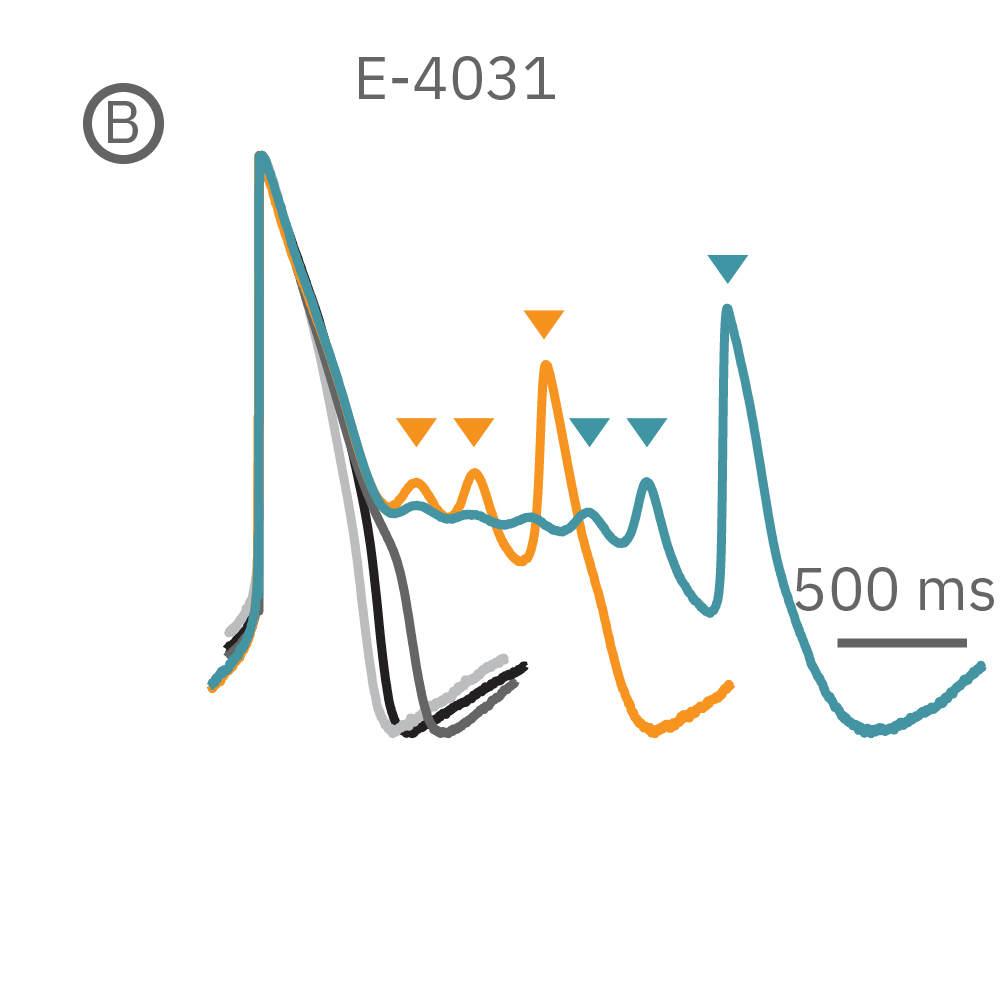
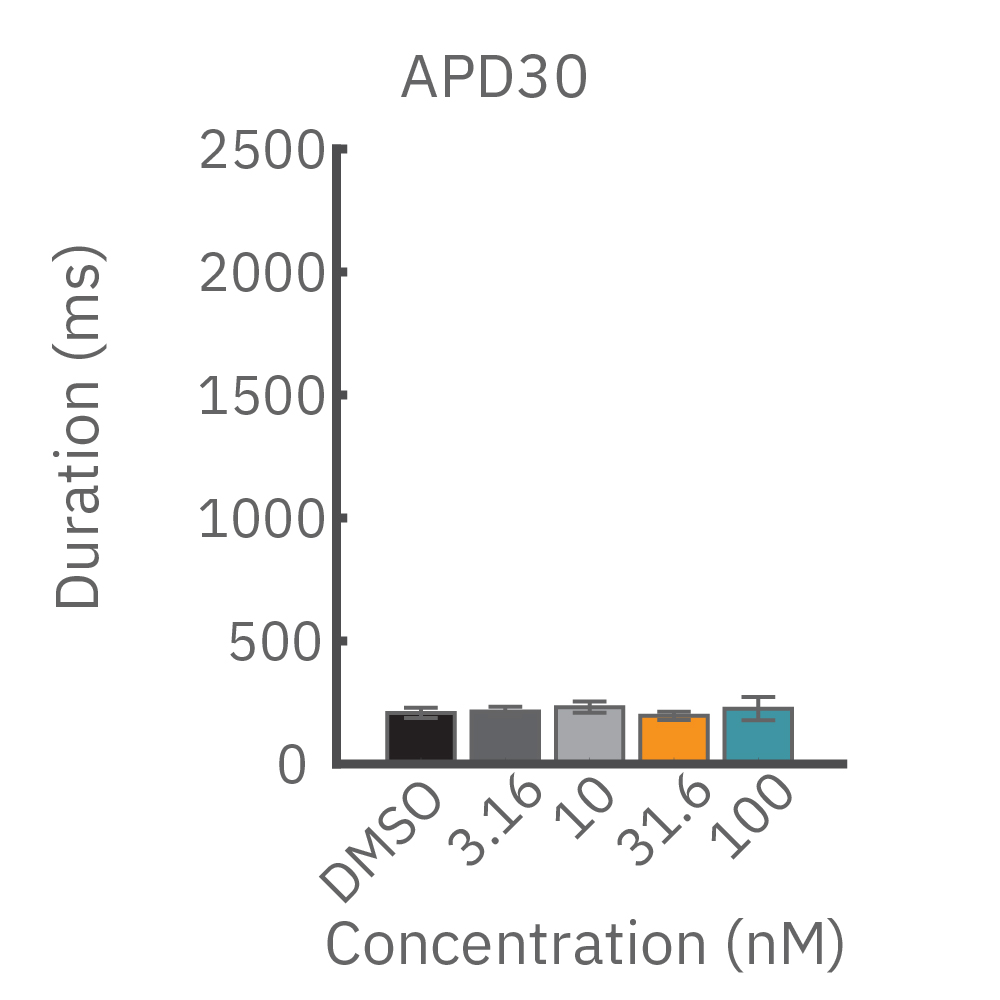
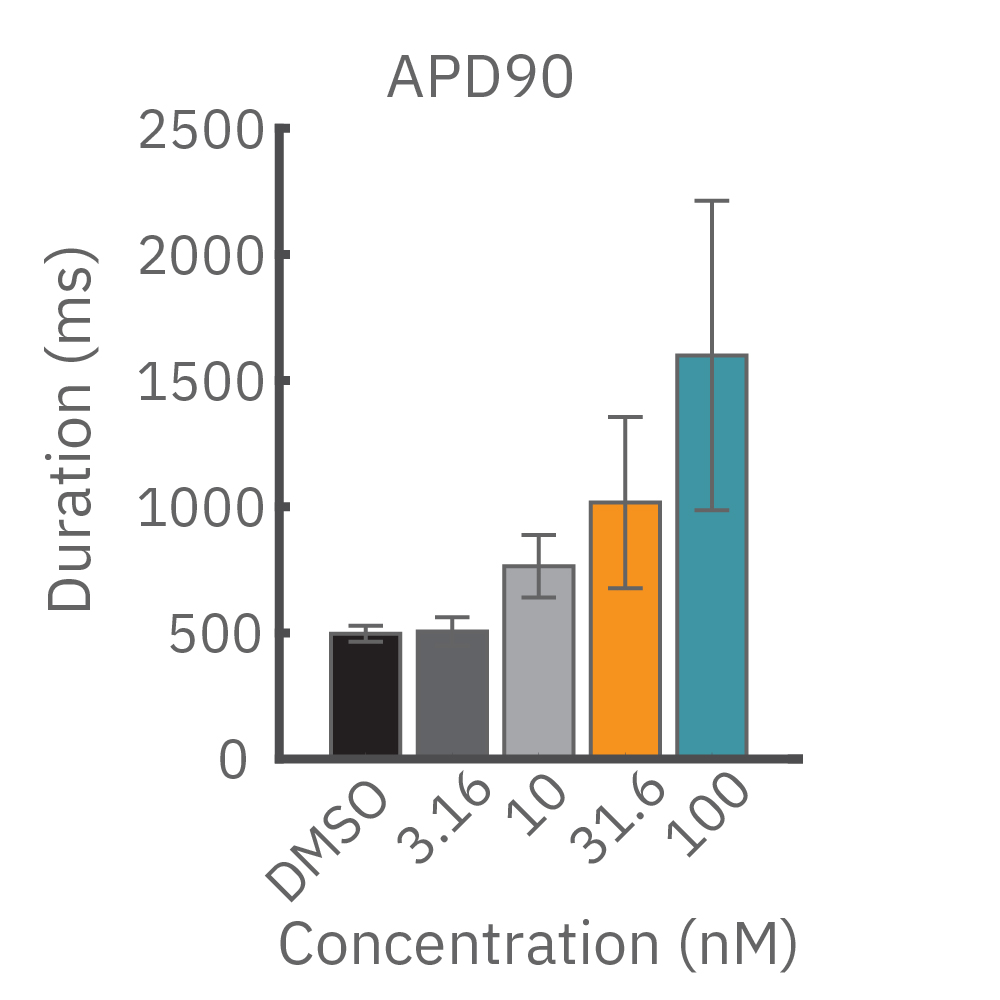
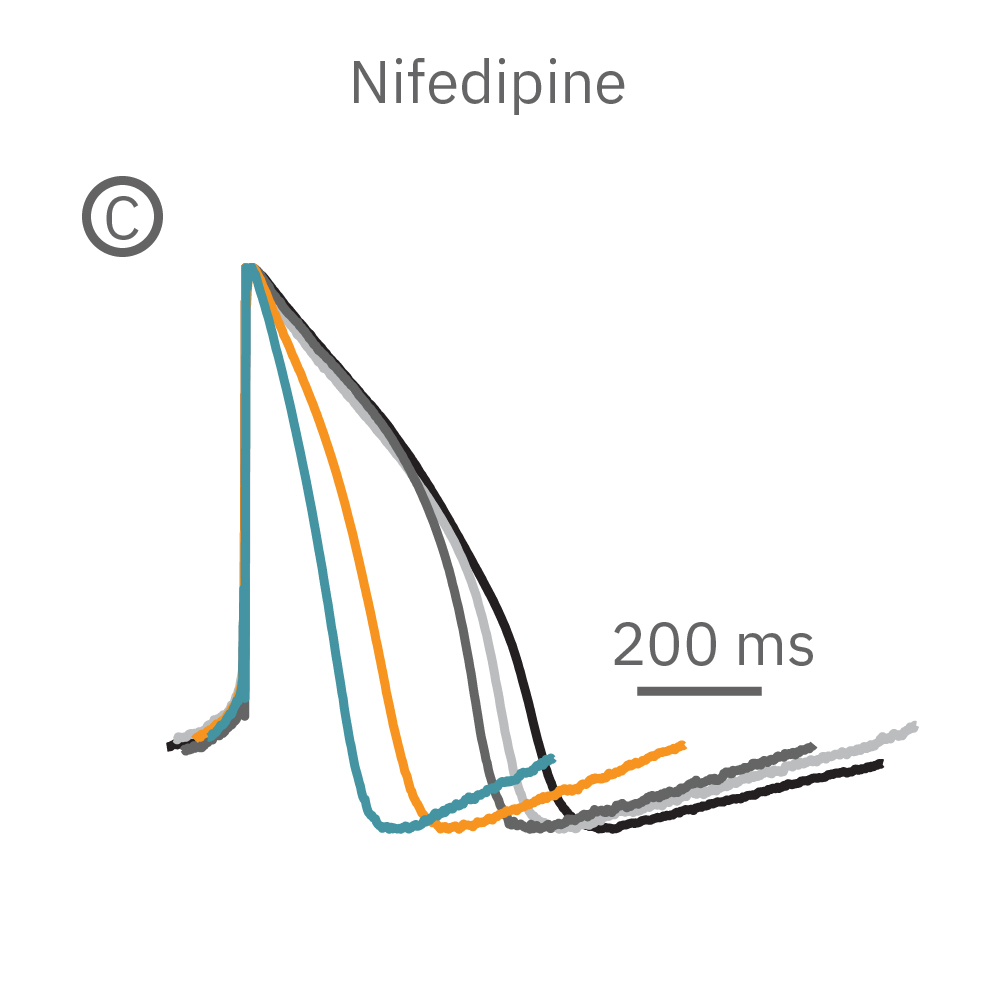
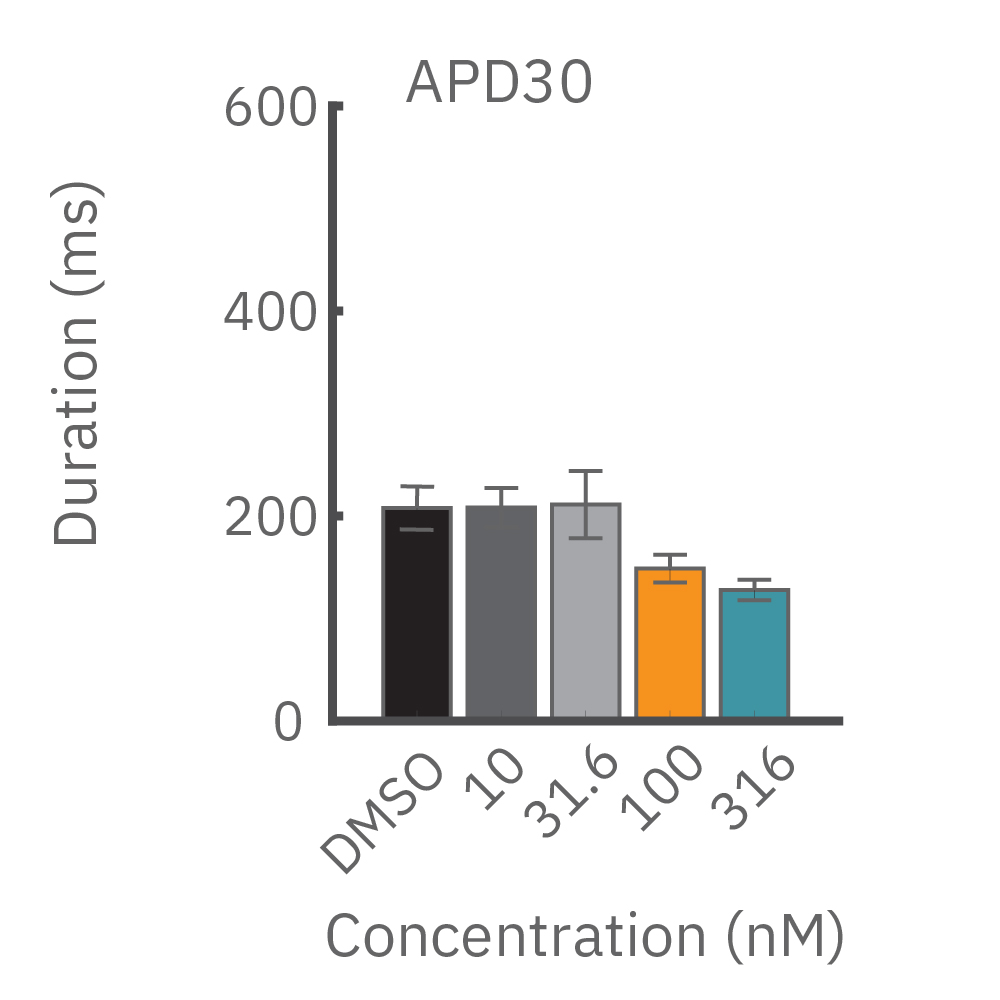
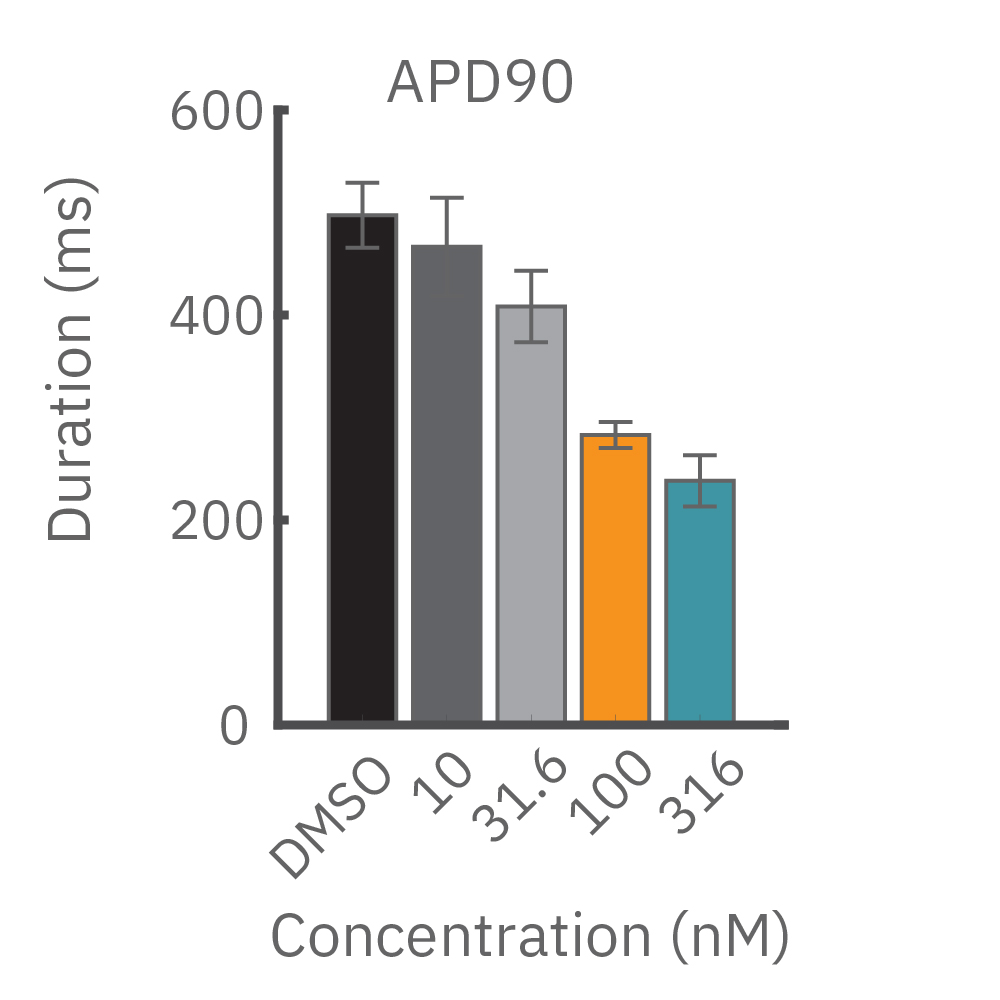
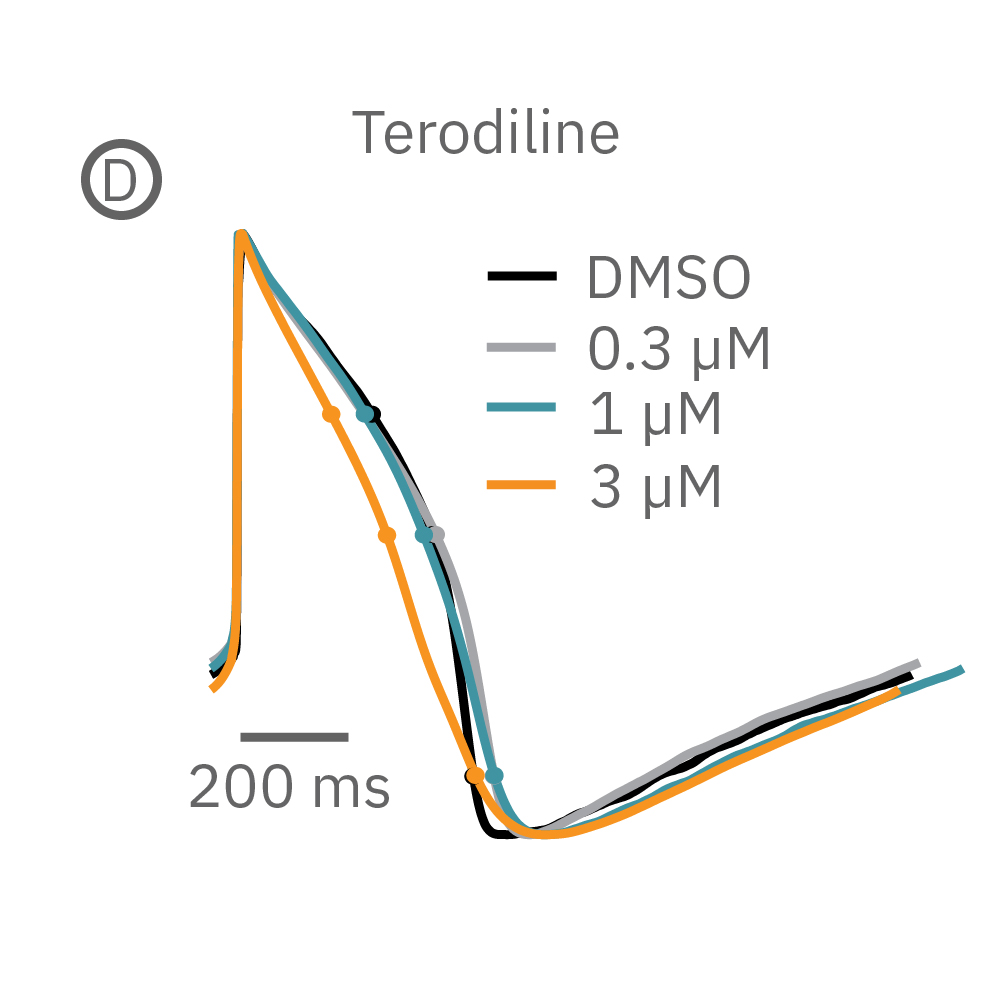

(B) E-4031 first prolonged repolarization and then generated repolarization irregularities. (C) Duration of the LEAP signal was shortened in a dose-dependent manner. (D) Triangulation was induced at higher concentrations, consistent with patch clamp results

Field Potential >>
How does it work?
The recorded cardiac field potential signal arises from the propagation of the cardiac action potential across the functional syncytium, much in the same way the clinical ECG arises from the propagation of the cardiac action potential across the heart. The field potential signal has clear markers for depolarization and repolarization enabling the quantification of important beating parameters.
What can you measure?
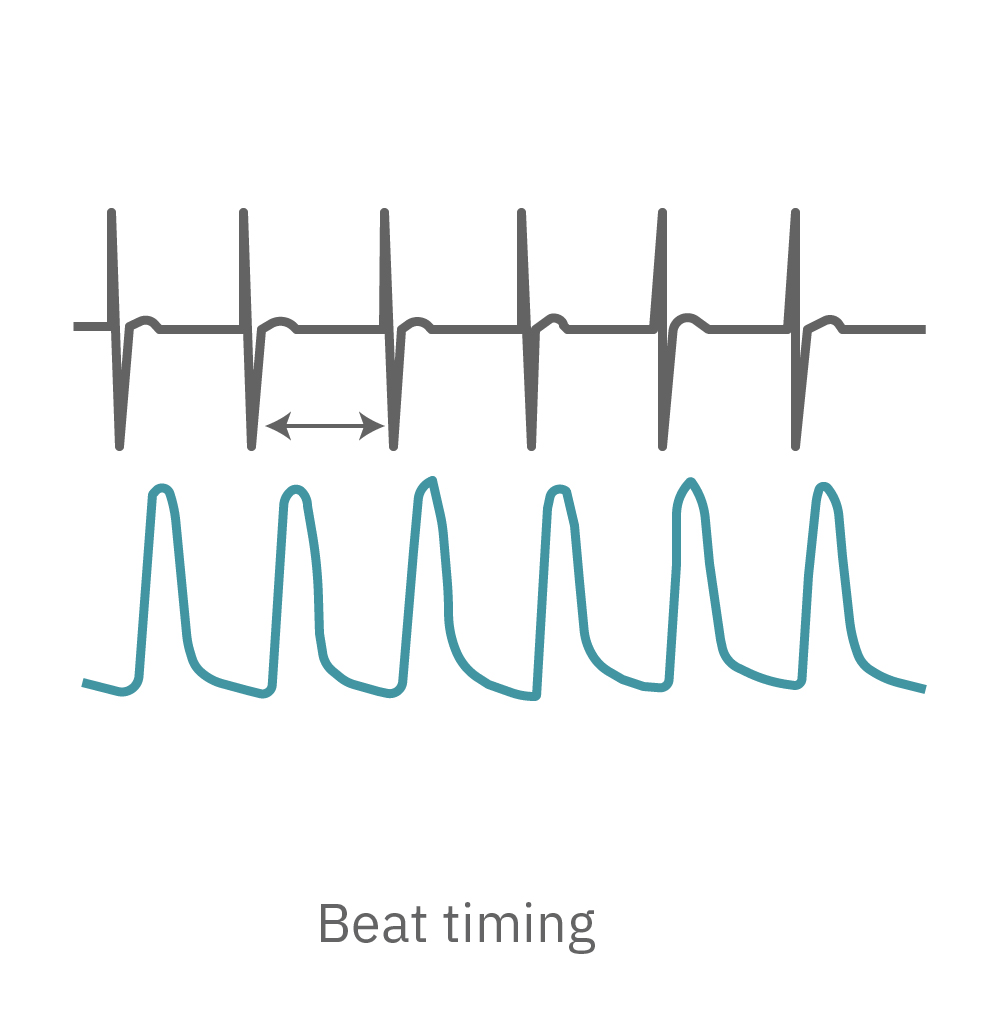
The Maestro system detects key parameters of cardiomyocyte activity, including depolarization, repolarization (FPD), beat timing, and irregular beating (arrhythmia).
Field potentials are gold standard for in vitro drug safety
The sensitivity of the Maestro MEA hiPSC-CM field potential (FP) assay is the result of years of development alongside CiPA, with a significant focus on accuracy and reliability, leading to the industry-leading performance of the Maestro MEA in the CiPA Phase I and Phase II studies (see link for more details). The label-free, noninvasive signal acquisition and precise environmental control enables quantification of acute or chronic responses.

Propagation >>
How does it work?
In the human heart, a coordinated contraction is required for efficient circulation. Slowed or disrupted conduction can lead to irregular heart beats, known as arrhythmia, making conduction a key component of cardiac assessment in vitro. When cardiomyocytes are cultured in MEA plates, each beat is initiated in one part of the culture and propagates across the monolayer.
What can you measure?
The Maestro system detects key parameters of the cardiomyocyte beat propagation, including conduction velocity, and determines the propagation pattern for each beat in order to report metrics describing the consistency in beat propagation.
Propagation quantifies cell-to-cell coupling
Pharmacological agents and cardiac disorders can affect conduction by 1) modifying the excitability of the cells, or 2) altering the gap junction coupling between cells. Paced assays are able to resolve subtle changes in conduction velocity, whereas gross conduction changes appear as inconsistencies in the propagation patterns.
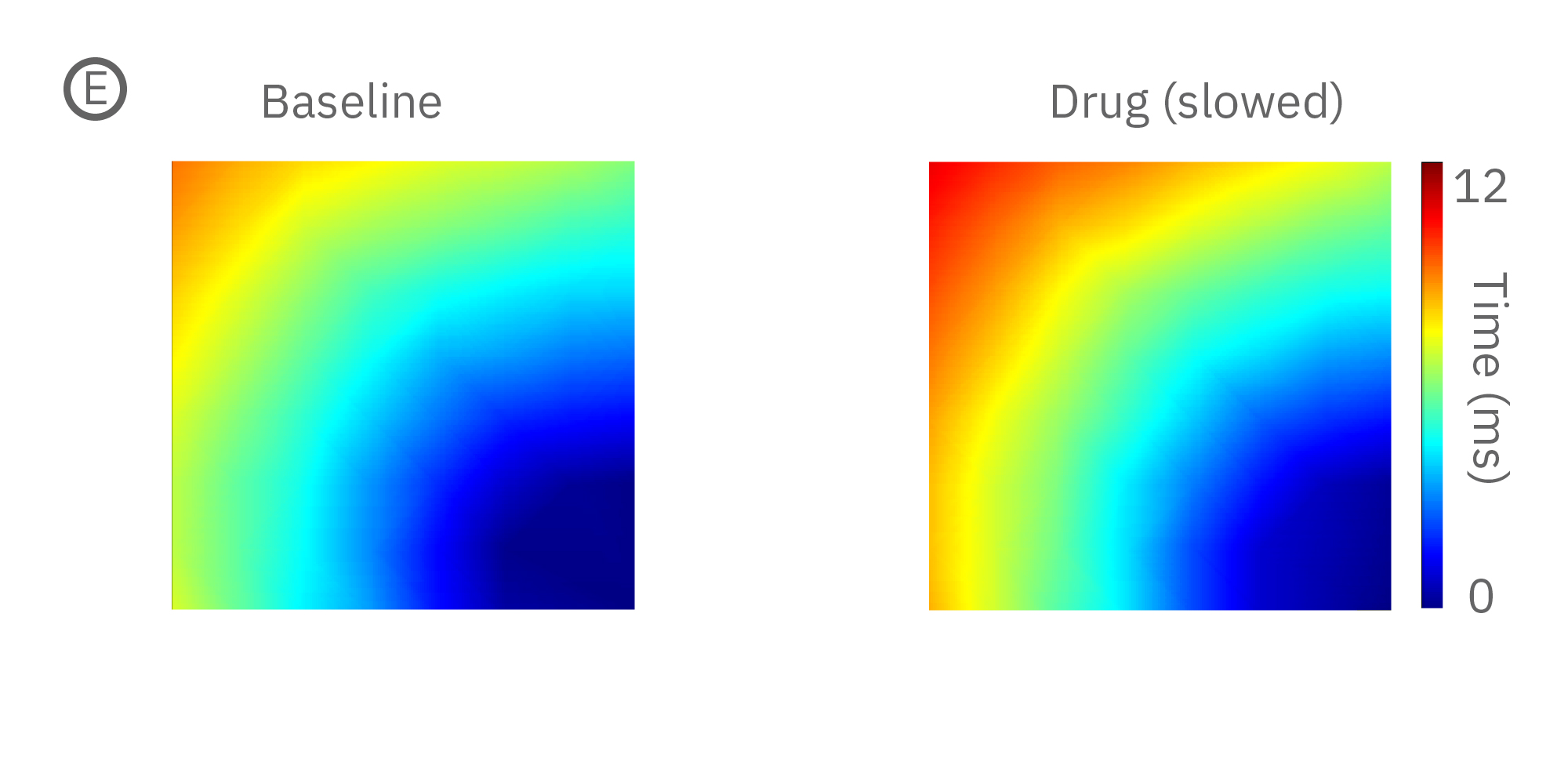
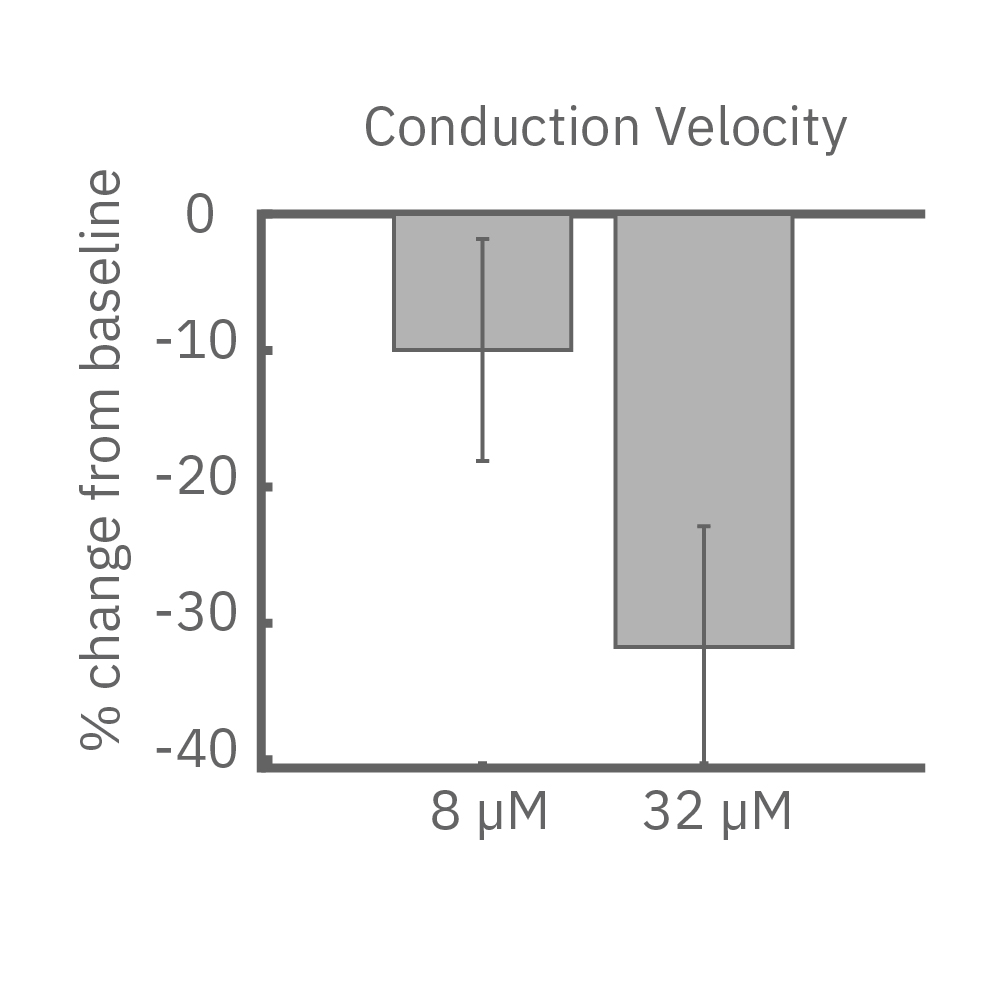
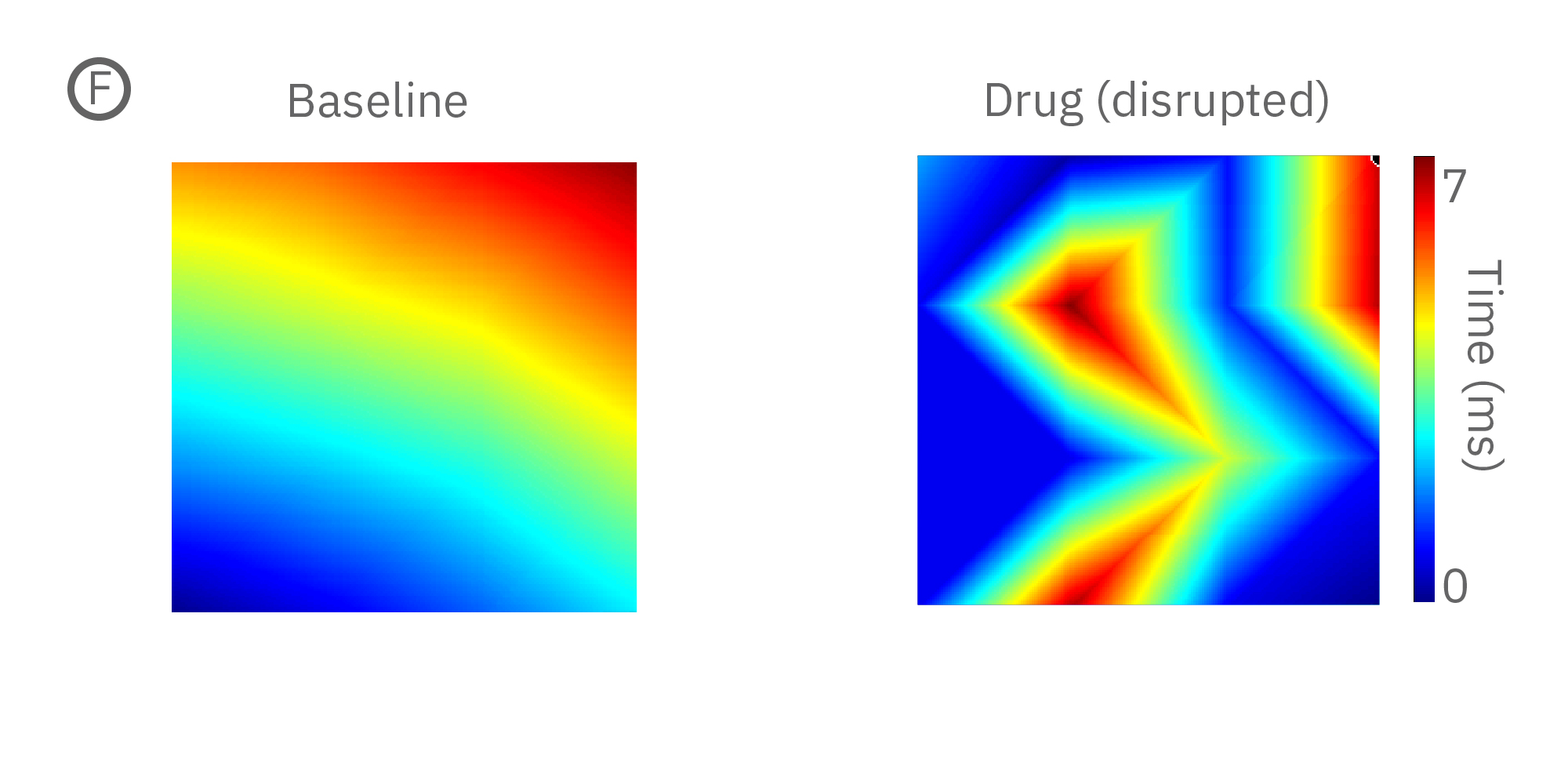
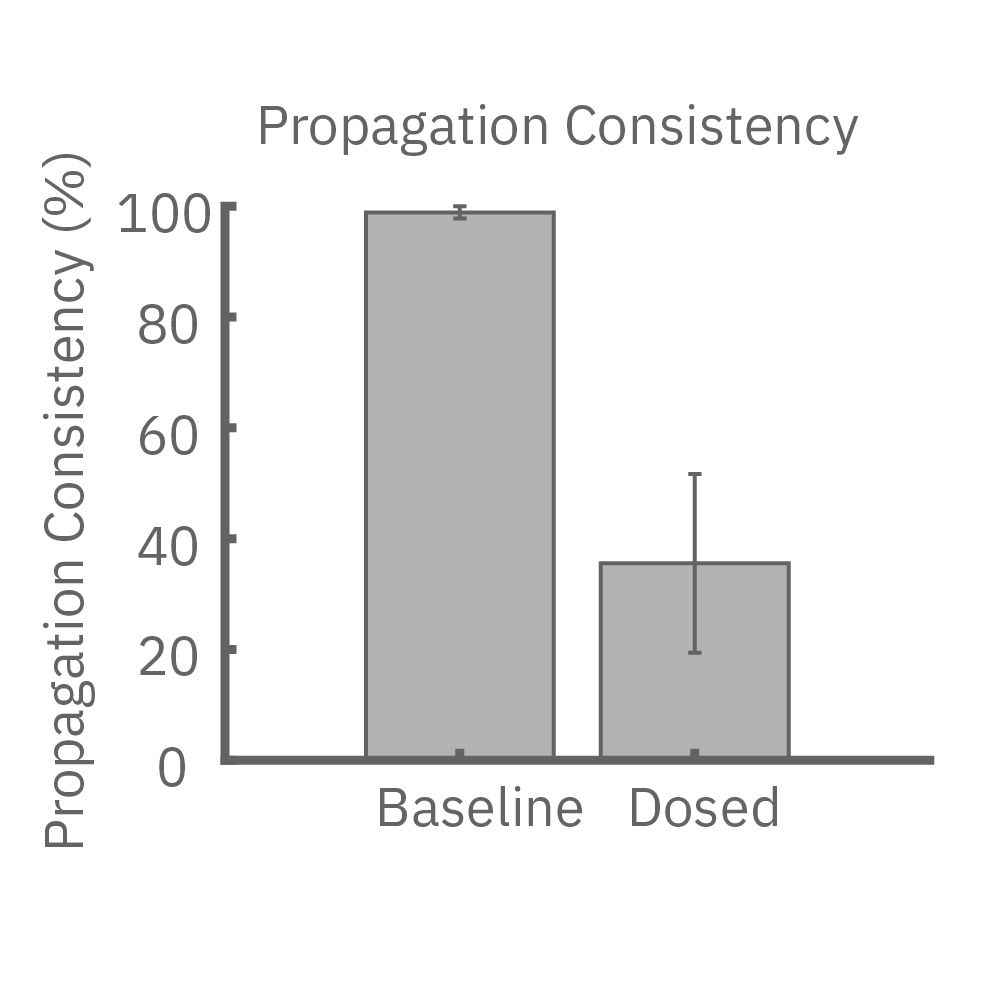
(E) Lidocaine, a Na+ channel blocker, decreased conduction velocity in hiPSC-CMs. (F) An anti-cancer drug caused a disruption in propagation pattern, and thus a decrease in propagation consistency from beat to beat

Contractility >>
How does it work?
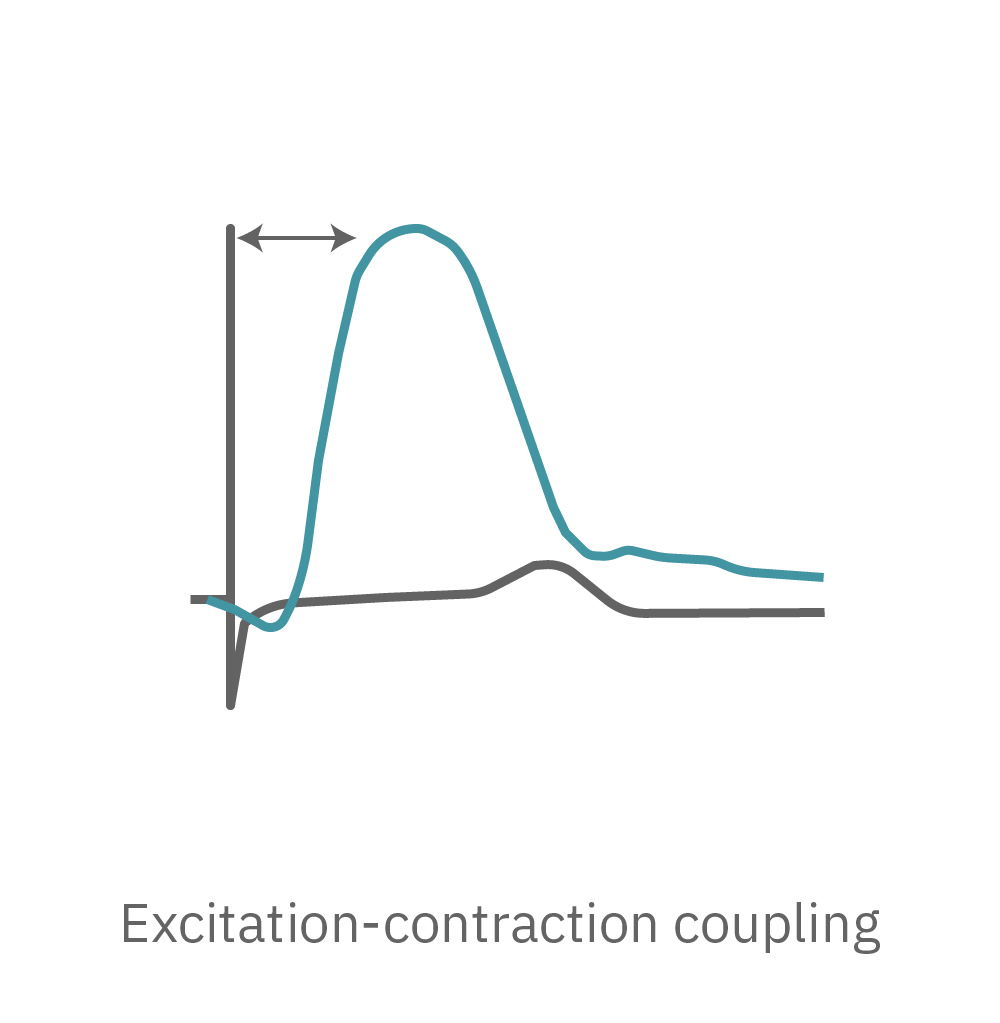
Cardiac excitation-contraction coupling describes the series of events from the production of an electrical impulse (action potential) to the contraction of muscles in the heart. As the cardiomyocytes mechanically contract and relax over a recording electrode, the shape change is detected as a respective increase and decrease in the impedance signal. An array of electrodes can map contraction and relaxation across the syncytium.
What can you measure?
The Maestro system detects key parameters of cardiomyocyte contractility, including beat amplitude, beat timing, and excitation-contraction delay.
Examine physical contraction in vitro
Contractility provides important information about cardiac beating. For example, blebbistatin, a myosin inhibitor, reduces the array-based cardiomyocyte contractility signal (A). At high concentrations, the cells are no longer contracting. Furthermore, array-based contractility enables advanced applications, such as recording from 3D cultures and robust contractility measurements despite variations in cell coverage across the well (B).
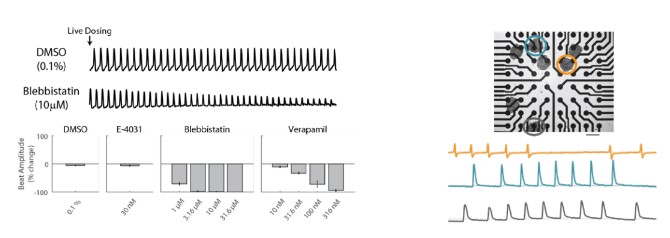
(Left) After dosing with blebbistatin and a vehicle control, blebbistatin rapidly reduced contractility relative to vehicle control. After 30-minutes, blebbistatin and verapamil showed clear dose-dependent reductions in beat amplitude, while vehicle control and E-4031 showed no change. (Right) Multiple cardiomyocyte spheroids in a well. Representative contractility recordings from 3 spheroids circled are shown.
How do you measure activity?
Evaluating electrical activity can give important context to your research. Detailed functional characterization is critical for understanding disease mechanisms, but many researchers avoid it because the techniques can be laborious. But electrophysiology doesn’t have to be difficult when Maestro microelectrode array technology makes functional readouts accessible. Why should you choose MEA?
The complete multiwell MEA system
Maestro MEA offers the best combination of throughput, resolution, and ease of use. Discover why Maestro MEA is emerging as the new standard for excitable cell function.

Detailed functional profiles
Dynamic spatial and temporal functional data reveals how cells fire and interact in culture
Noninvasive cell monitoring
Noninvasive electrodes track activity over time for maximum experimental flexibility

Easy assay technique
Requires only basic culture techniques with no labels, dyes, or complicated steps
Key features
- >> Multimodal configurations
- >> Simultaneous full-plate recording
- >> Built-in environmental controls
- >> Powerful, intuitive software
- >> Automation compatibility
- >> Noise-reduction engineering
- >> Barcode plate tracking
Our commitment to our customers
With over 15 years of experience bringing innovative new products to our customers, we strive to accelerate your research by making live, functional biology more accessible. Our design philosophy is to ensure all of our products are:
Flexible
Hardware designed for broad, integrated functionality in one instrument
Easy to use
Intuitive instruments, consumables, and software for fast, easy adoption
Smart technology
Easy to run with no complicated steps, saves time and money.




