候補化合物や治療法の効能及び安全性評価において、in vitro ライブセルへの効果検証は不可欠です。細胞毒性(傷害)、細胞生存 (viability) アッセイは多種存在しますが、それぞれに以下のような課題が提起されます。
- 染色剤や試薬などによる毒性の排除
- エンドポイントアッセイにおけるアッセイタイミングの選定。
- アッセイの煩雑さとコスト
Maestroによるインピーダンスアッセイは、細胞の傷害、生存率の変化などを、ラベルフリーで経時的に測定します。アッセイの繰り返しも不要で、一度の簡単な測定で細胞の変化の全容をとらえることが可能です。
細胞傷害、生存率変化を経時的にリアルタイムで測定
細胞の反応変化の検証には、エンドポイントアッセイが多く用いられます。エンドポイントアッセイは、反応直後の1点のタイムポイントの結果を得るには有用ですが、細胞の傷害、生存率の変化など状態変化の検証にはアッセイの繰り返しが必要で、コストと手間がかかります。
Maestro によるインピーダンスアッセイは、1枚のプレートによる1度のアッセイで、細胞死のカイネティクスを検証することが可能です。(インピーダンス測定に関する詳細はこちらを参照下さい。)
Maestro による細胞生存性アッセイ –エンドポイントアッセイ との比較-
インピーダンスアッセイでは、インピーダンス値の変化による細胞の生死の定量化が可能です。その相関の精度は高く、ISO 20391-2 で定められた細胞カウントガイドラインにおいても適合が得られました。以下の事例では、Maestro による細胞カウントをエンドポイントアッセイ(MTT) と比較しています。
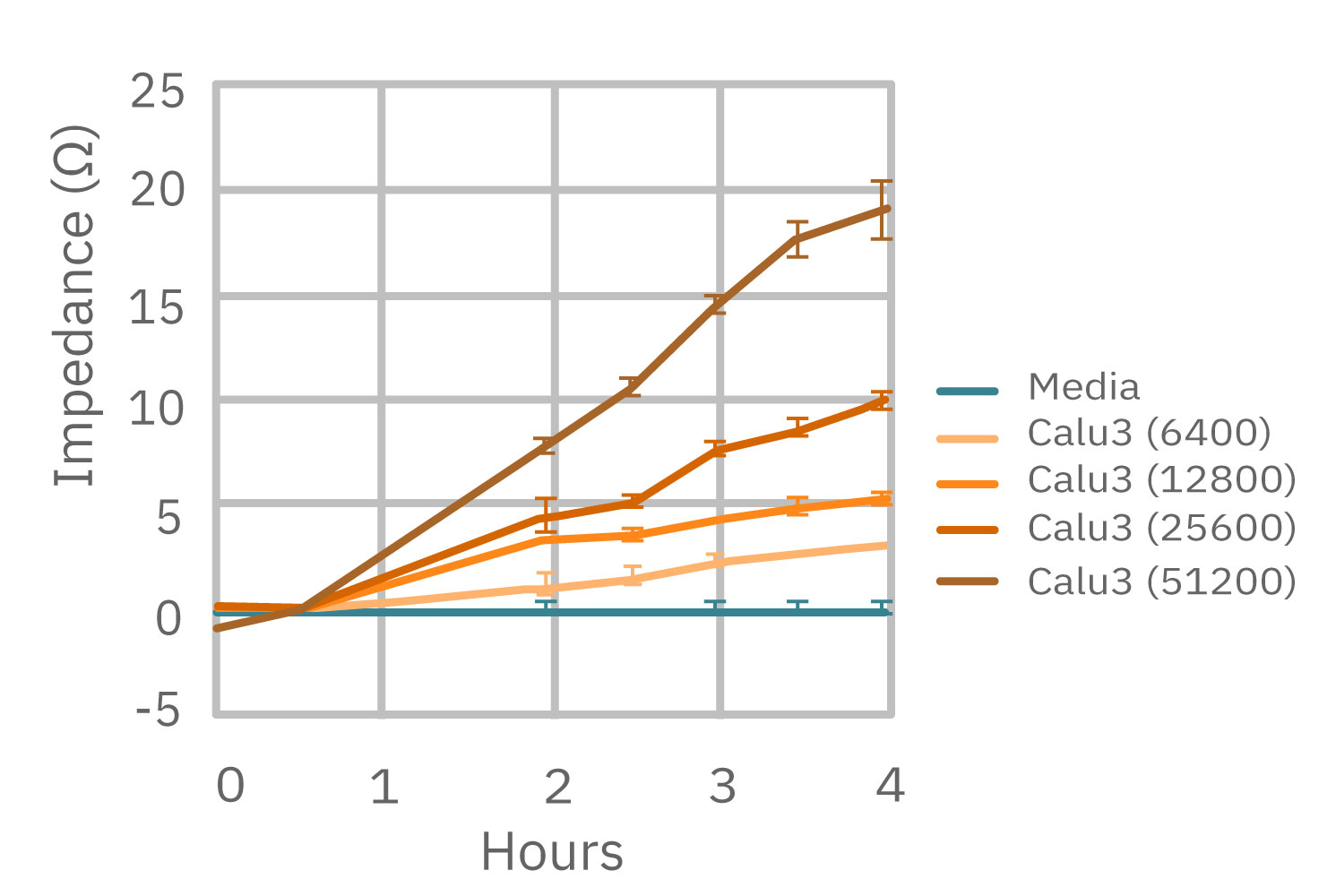
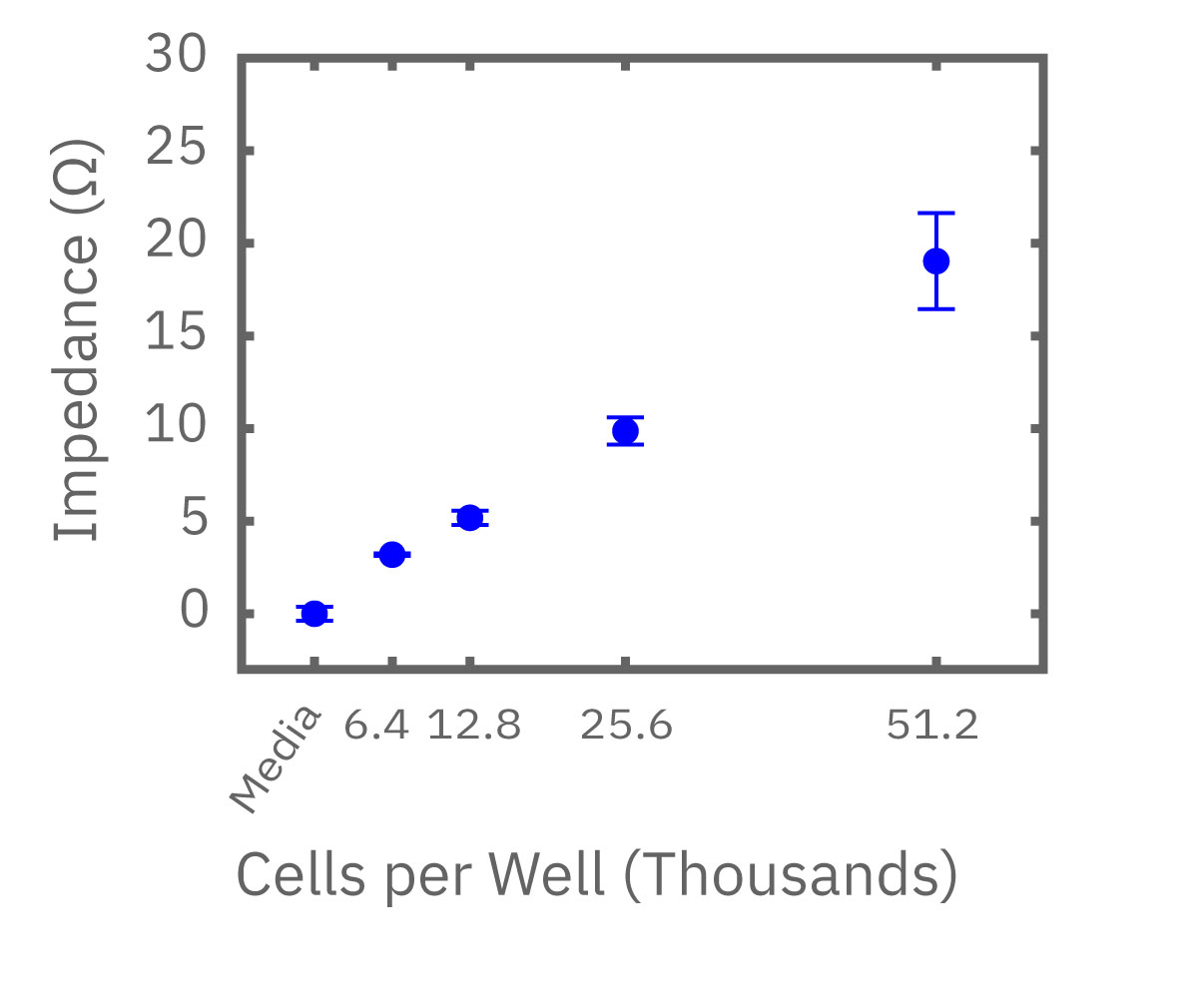
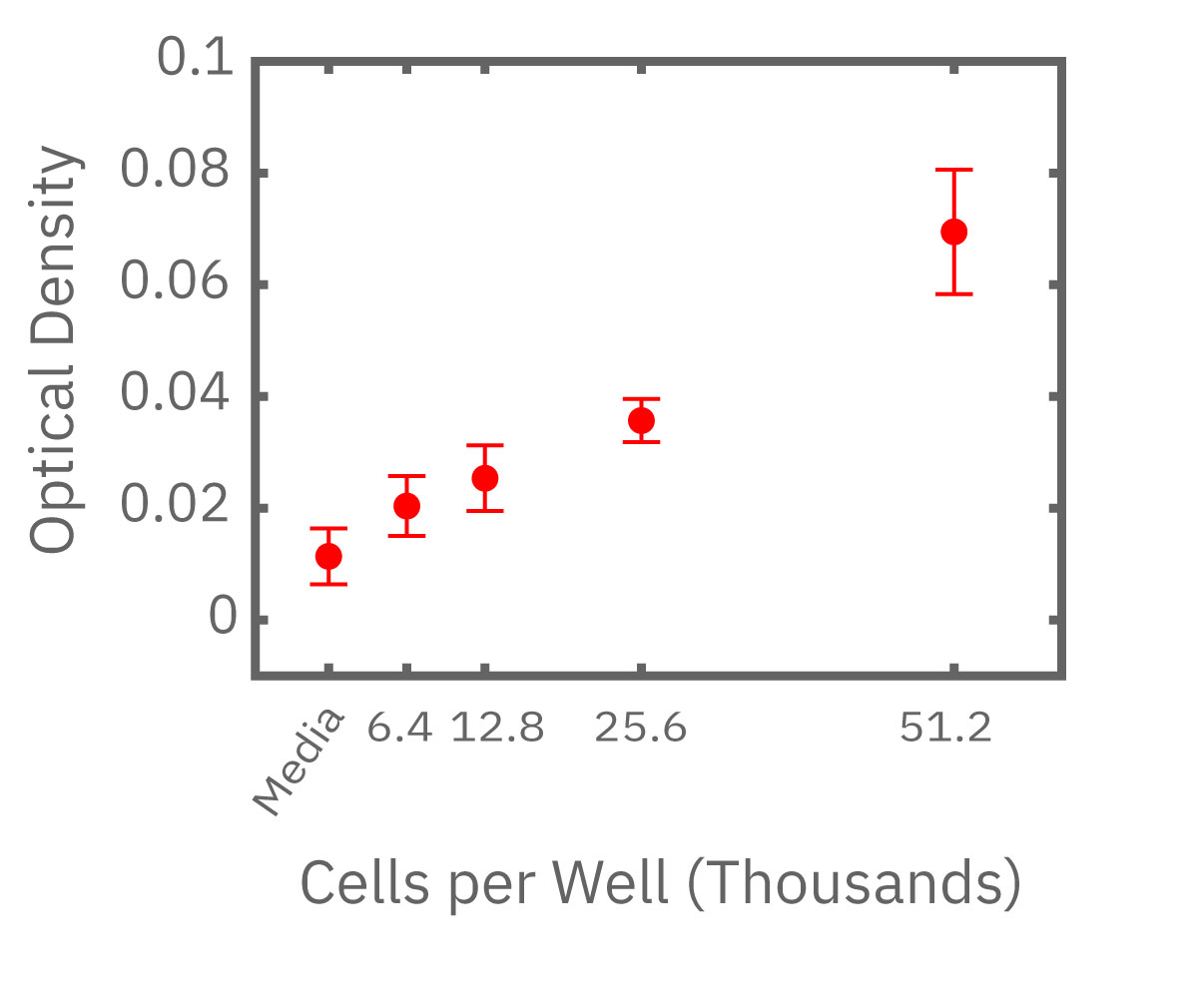
(左図) Calu-3 細胞をプレート上に6400 – 51200 細胞/well の異なる密度で播種し、Maestro でインピーダンスを測定した。図は各細胞密度毎の、4時間に渡るインピーダンスの経時変化を示す。 (中央図・右図) 中央図は細胞播種から4時間後の各細胞密度毎のインピーダンス値を示す。MMTアッセイで得られた結果(右図)と相関が得られた。
抗がん剤による細胞傷害 (Cytolysis) とkill time 50% の算出
本事例では、抗がん剤によるがん細胞の傷害(毒性) を数日間に渡り評価しました。経時的な測定により傷害(細胞死)の割合 (Cytolysis) を算出し、更に Kill time 50% (細胞死50%までの到達時間)を検証しました。
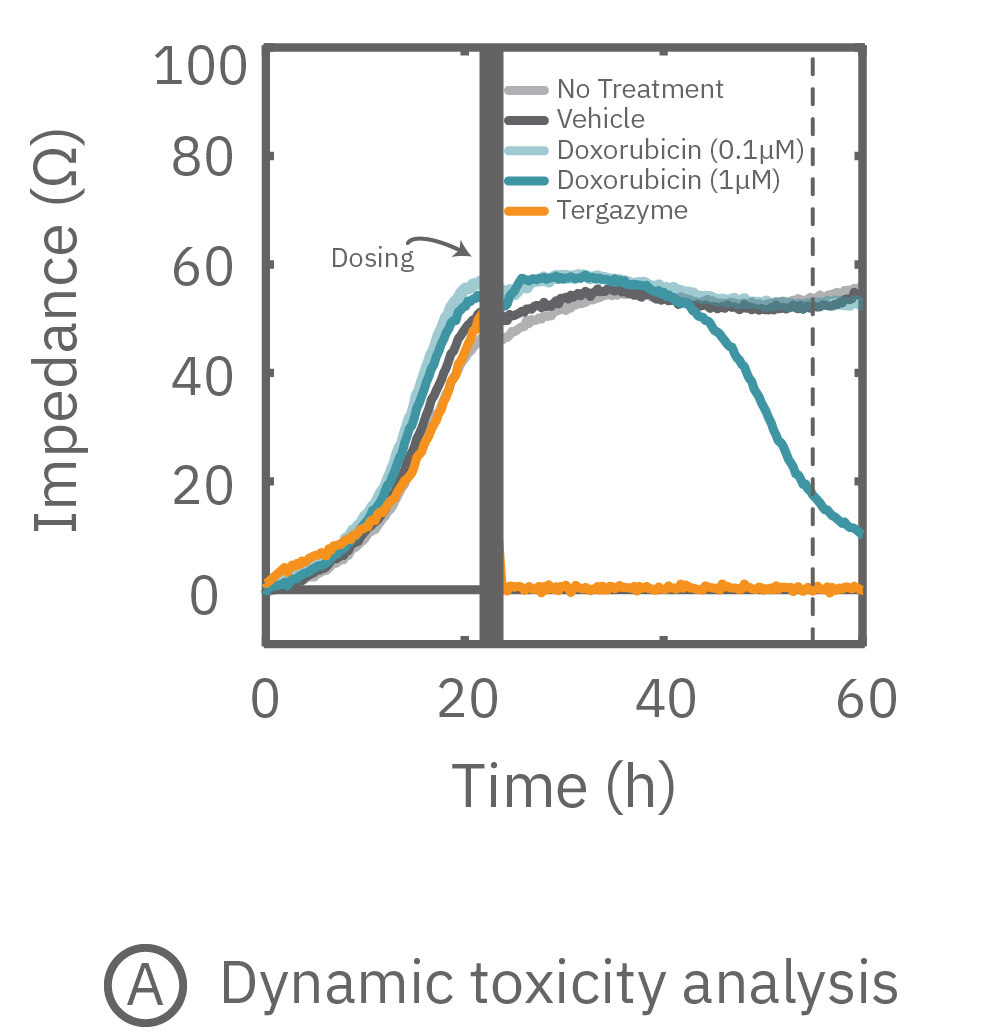
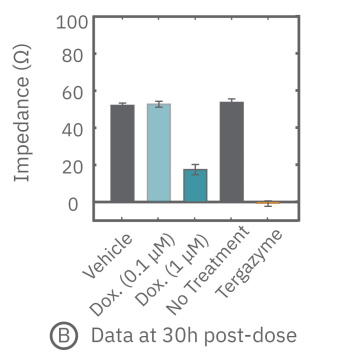
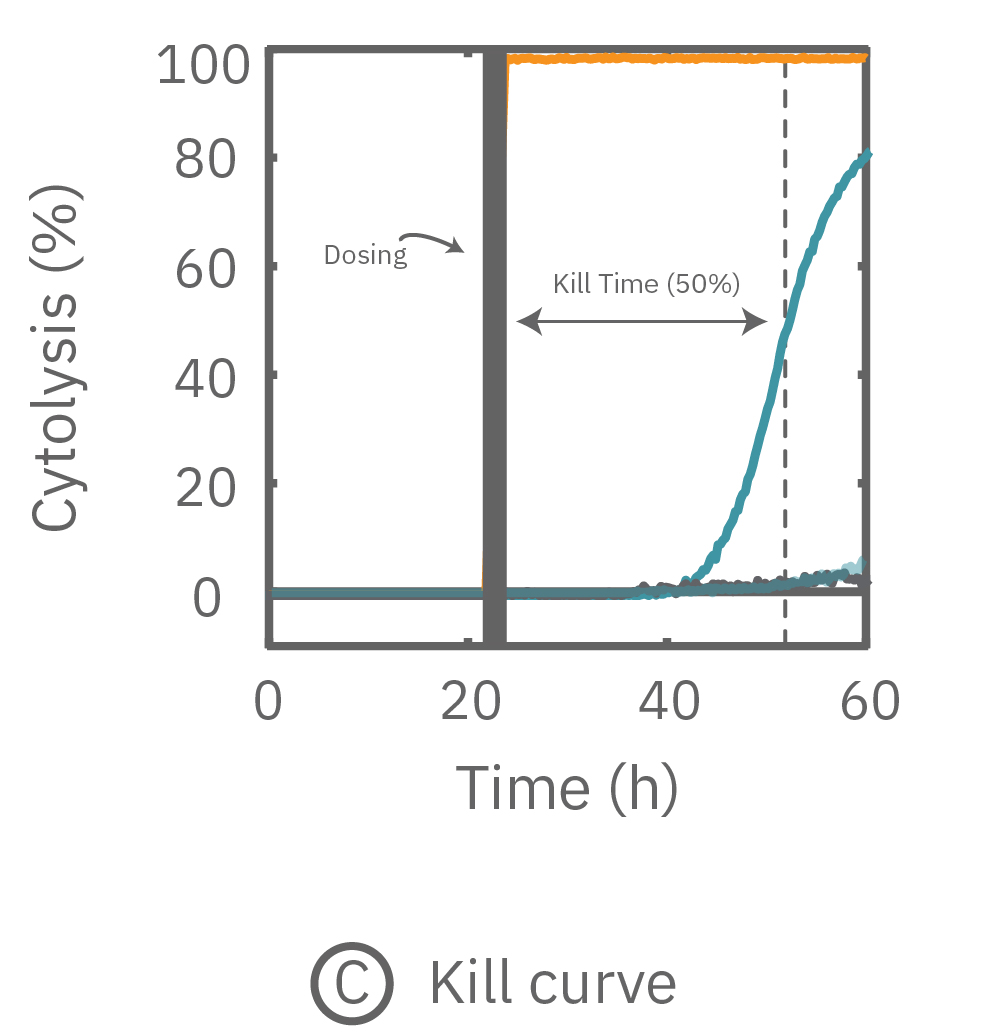
(A) プレート上にがん細胞を培養しインピーダンスを測定した。24時間後、1部の well にドクソルビシンを投与し、さらに 36 時間に渡ってインピーダンスを測定した。ドクソルビシン投与 well のインピーダンス (水色) は減少した。
(B) ドクソルビシン投与から30時間後のインピーダンスを示す。1μM投与においてインピーダンスの減少が得られた。
(C) 薬剤非投与 well のインピーダンスを0%、テルガザイム投与を100%として算出されたCytolysis。投与後約31時間でkill time 50%に到達した。
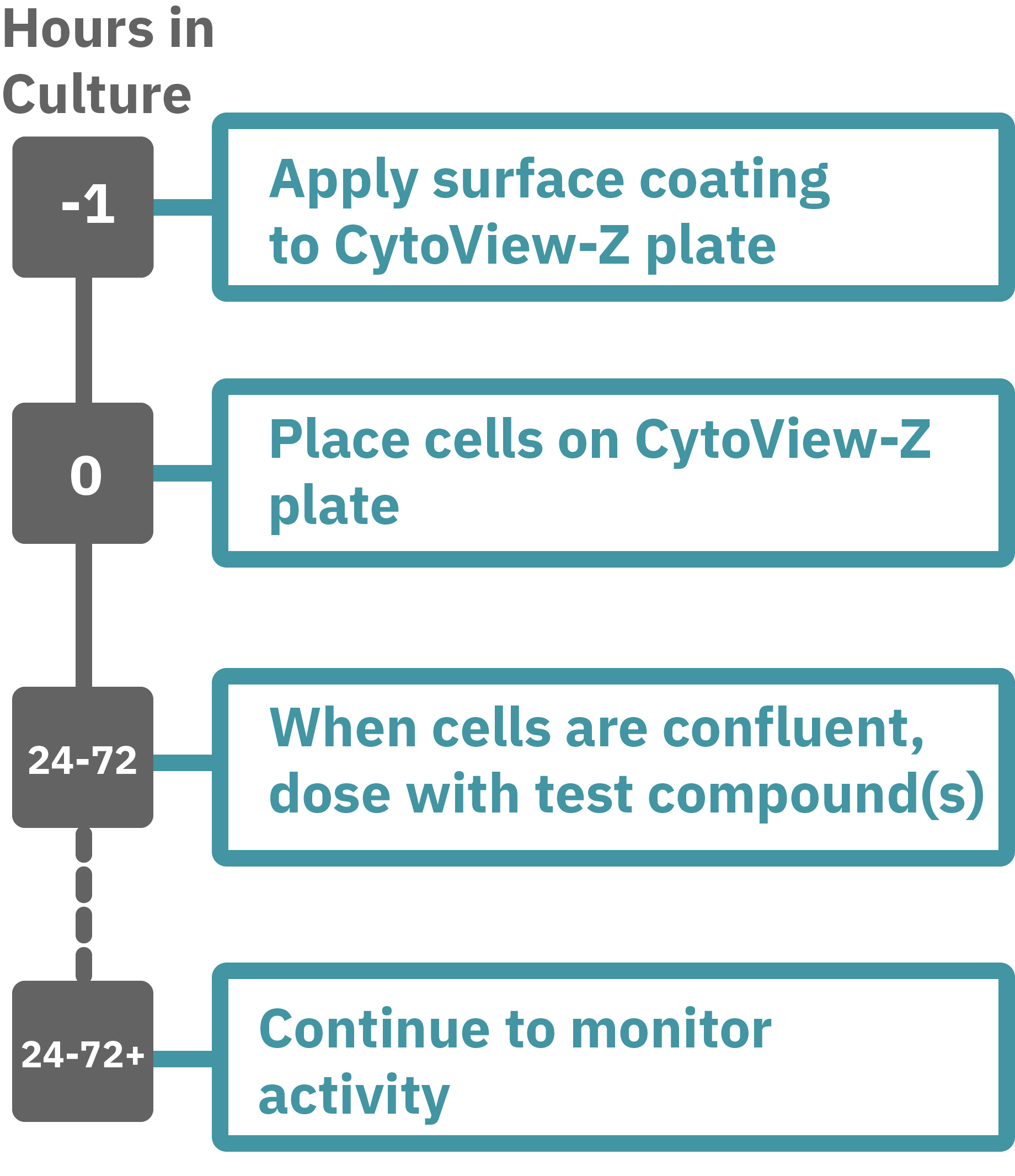
Maestroによる、インピーダンスアッセイはとても簡単です。事前コーティングされた CytoView-Zプレート上に細胞を播種します (Hour 0)。Maestroシステムにプレートを搭載するとと同時に、温度・CO₂ 濃度制御とインピーダンス測定が開始されます。細胞の増殖・電極への接着に伴いインピーダンスが上昇します (Hour 0 ~ 24-72)。
細胞が Well 内でコンフル状態になったらテスト化合物を投与し、継続してインピーダンスを測定し、その変化により細胞の生存率を検証します。

Maestro Z/ ZHT, Pro/Edgeによる細胞傷害(毒性)アッセイ:特徴
-
経時的観察 - 細胞毒性(傷害)をリアルアルタイムで測定します。専用アプリで、実験室の外からでもライブデータの確認が可能です。
-
ラベルフリー - 平面電極によるインピーダンス測定は、染色・試薬などを必要としません。ラベルフリー測定で数日間に渡る測定・観察が可能です。
-
インキュベータ不要 - Maestroには温度・CO2濃度コントローラが内蔵されています。インキュベータ等の周辺装置は不要。安定した環境下で数日間に渡る連続測定が可能です。
-
細胞可視 - CytoView-Z 96 well プレート底面中央部は透明になっています。必要に応じて、細胞の観察が可能です。
-
培養から測定まで同一プレート使用 - アッセイの全行程を同一プレートで行います。他のハイスループット・プラットフォーム(例:フローサイトメータ)のような容器の入れ替えなどは不要。細胞への負担を最小限に抑えることができます。
-
スマートフォン・アプリ - 専用のスマートフォンアプリに対応しています。数日間に渡る細胞傷害の様子を、実験室の外からでも、リアルタイムに観察して頂けます。
-
簡単 - セミ・オートメーションシステムです。ハードウエアの操作はボタン1つ。専用のソフトは、インピーダンスの変化をリアルタイムで表示します。解析結果のエクスポートも容易です。

Impedance
Show Full DetailsWhat is impedance-based cellular analysis?
Impedance-based cell analysis is a label-free, real-time technology that monitors live cells by measuring how electrical signals change across microelectrodes embedded in the culture surface. As cells attach, spread, and respond to treatments, the Axion BioSystems' Maestro Z impedance platform provides dynamic, continuous cellular profiles — capturing both baseline behavior and drug- or immune-driven effects.
Why does real-time, label-free monitoring matter?
Traditional assays often rely on dyes, labels, or destructive endpoint measurements, which can interrupt normal biology and miss transient responses. Impedance monitoring solves this by delivering continuous, noninvasive functional readouts — enabling researchers to observe biological change as it happens, particularly in drug discovery, toxicology, and disease modeling.