What are the advantages of MEA to study in vitro glioma and glioblastoma models?
Glioma brain tumors originate in glial cells including astrocytes, oligodendrocytes, and ependymal cells, with glioblastomas being the most common and aggressive type of malignant glioma. Rapid scientific advancements in neuro-oncology are advancing our understanding of their complex cellular interactions and genetic mutations, leading to the development of new immunotherapies, including promising CAR T cell therapies.
Axion’s live-cell assay platforms offer non-invasive, real-time analysis of cellular interactions providing unparalleled insight into the mechanisms of cancer and the development of potential interventions.
Brain tumor research with in vitro models
New in vitro models are developed each day and the right tools to measure complex cellular interactions and neuronal activity can accelerate the research into brain cancer.
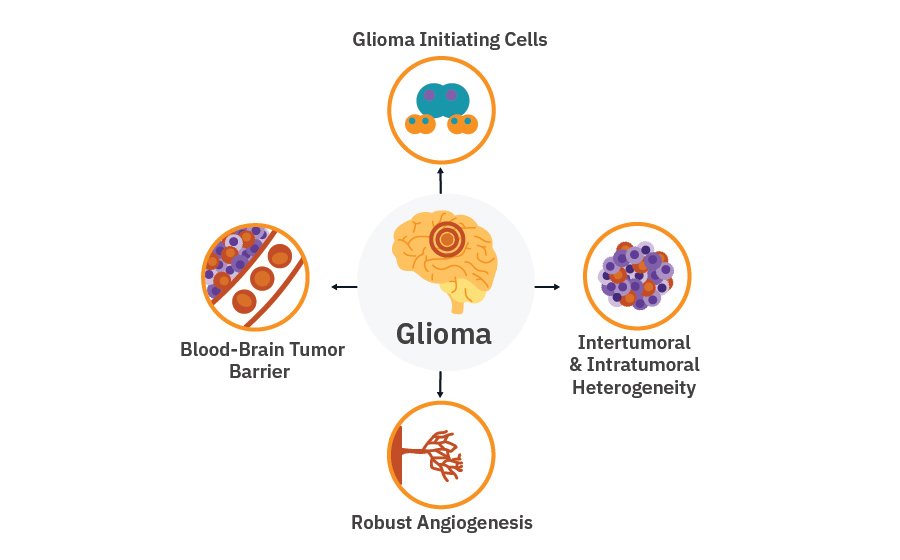
Gliomas differ from other solid tumors due to:
- Robust angiogenesis
- High level of intertumoral and intratumoral heterogeneity
- Blood-brain tumor barrier
- Glioma initiating stem cells
See how researchers use our tools to accelerate immunotherapy discovery and study the effects of neuron-glioma cell interactions.
Neuro-oncology research with in vitro models of glioma
-
Glioblastoma-mediated remodeling impacts functionality of human neural circuits>
-
In vitro assessment of CAR T cell potency reveals cytotoxic effects toward U87MG glioma cells>
-
Isocitrate dehydrogenase mutation promotes the development of glioblastoma-induced seizures in vitro>
Purpose: To investigate the mechanisms underlying the glioma-induced remodeling and hyperexcitability of neural networks. It has been established that brain tumors, and especially gliomas, can affect patients' cognitive and physical functions.
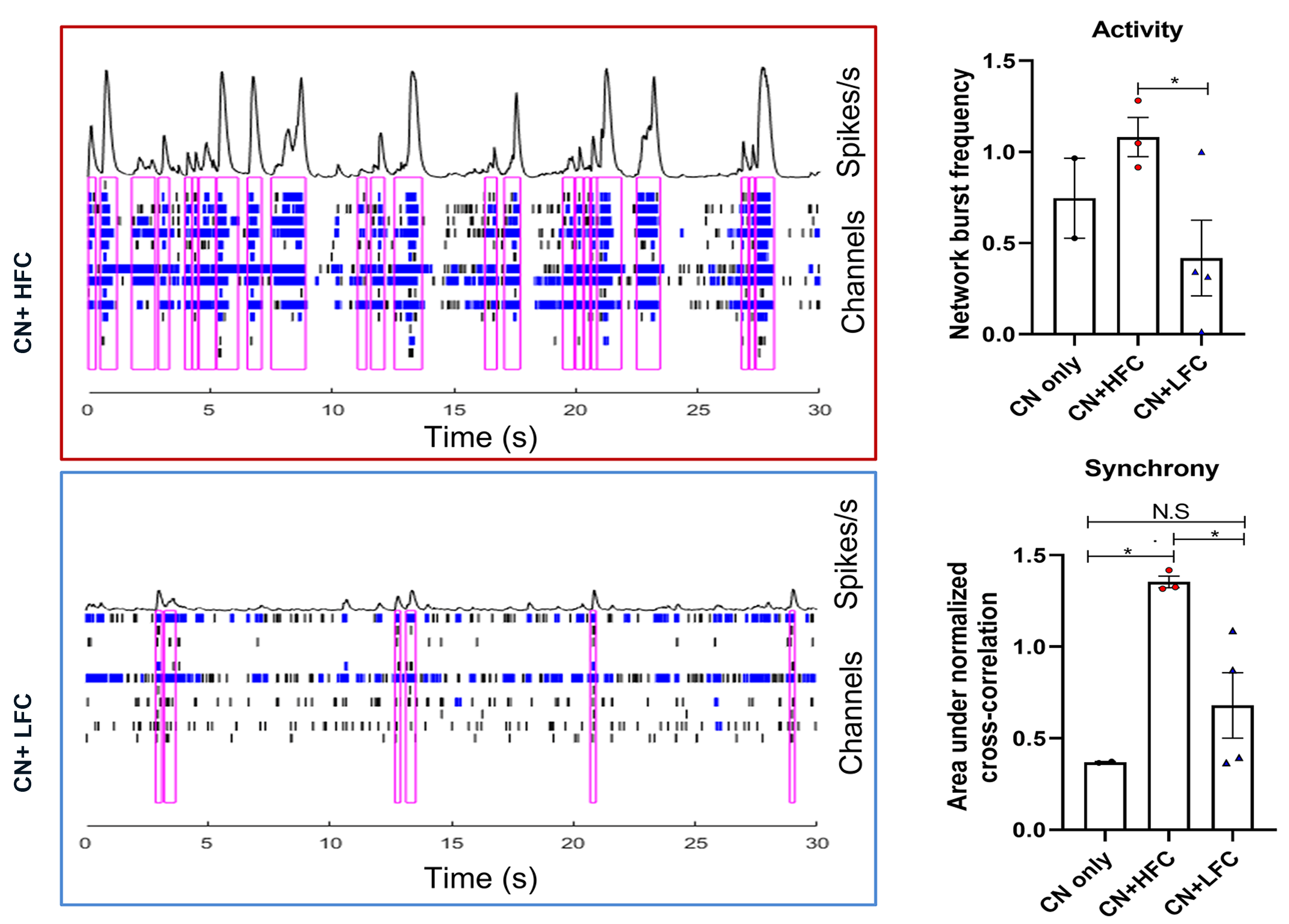
Primary patient-derived glioma cultures, from tumor regions with high (HFC) and low (LFC) functional connectivity to the rest of the brain, were co-cultured with cortical neurons, and the electrophysiological responses of these co-cultures were assessed using the Maestro MEA platform.
Result: Maestro MEA data demonstrated that co-cultures with HFC tumor cells were more active and synchronous than co-cultures with LFC tumor cells, suggesting that HFC tumor cells enhance remodeling of neuronal connectivity [Krishna 2023].
Purpose: To investigate the targeting potency of activated human CAR T cells on U87MG glioma cells. Glioblastomas are difficult to treat and identifying effective treatment strategies is crucial.
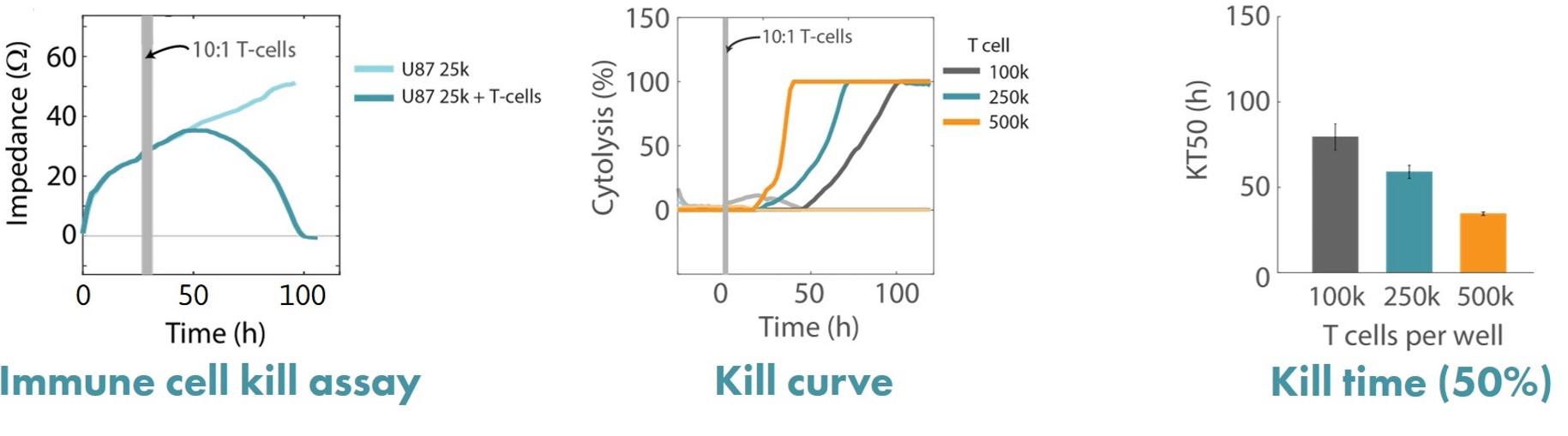
Human glioblastoma U87MG cells were cultured at different seeding densities and treated with activated CD3/CD28 human T cells. Real-time cytotoxicity was measured and kinetic KT50 values were calculated using continuous impedance monitoring performed by the Maestro Z platform.
Result: Maestro Z data demonstrated that the addition of activated CAR T cells resulted in T cell-mediated lysis of U87MG cells, with higher seeding densities of cancer cells resulting in faster cell lysis by proportionally seeded T-cell. [Karumbaiah, 2020].
Purpose: To investigate the role of isocitrate dehydrogenase (IDH) mutation, which is common among epileptogenic gliomas. Glioma-related seizures occur in more than 70% of patients and may result in a severe deterioration of cognitive function.
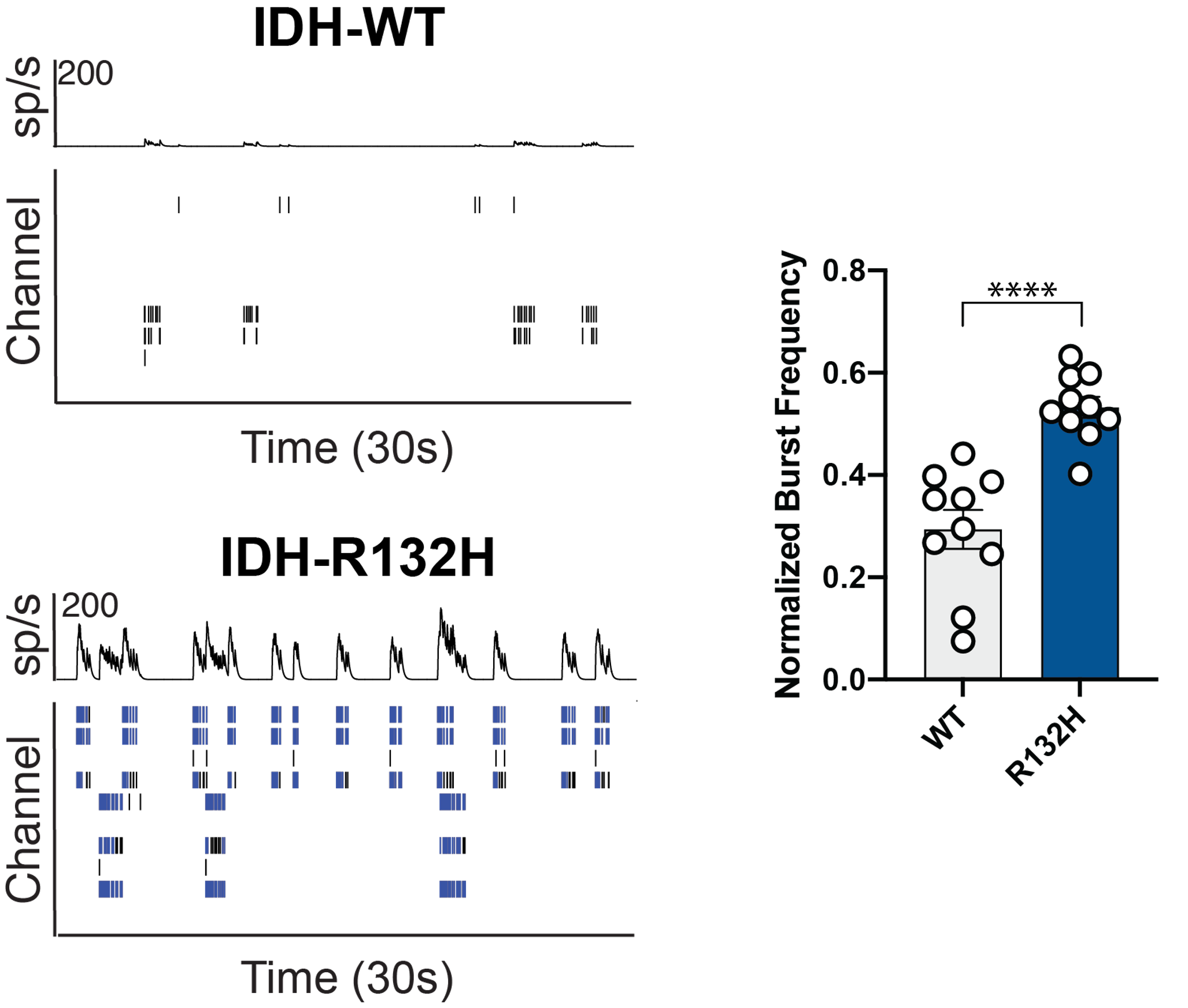
Primary rat cortical neurons were co-cultured with glioma cells with or without an IDH mutation and analyzed using the Axion’s MEA platform.
Result: Maestro MEA data demonstrated that in the presence of glioma cells carrying the IDH-R132H mutation, neurons showed an increase in neuronal bursting activity [Mortazavi 2022].
“Great results! Very excited to do more with my cancer model with the Maestro Edge”
Easy to use and gives you TONS of data rather quickly. Easily able to monitor spontaneous activity and evoked activity from sensory neurons. Very cool that we can do this in our cancer model so easily. Can't wait to investigate these sensory neurons after we treat mice with difference chemotherapeutics.
- Caitlyn Gaffney. MD Anderson Cancer Center, Texas, USA

FAQ:
- The Maestro MEA platform offers a controlled environment for studying detailed neural network activity in vitro.
- High-throughput multiwell plates make it ideal for screening patient-specific lines and therapeutics.
- Noninvasive monitoring allows for the study of long-term effects and disease progression.
- It is easy to use, requiring only basic cell culturing techniques to measure neural electrophysiology.
What kind of neural cultures can be measured on the Maestro MEA?
The main requirement is that you have electrically active cells. Primary or stem cell-derived neurons can be used. They may be cocultured with our without glial cells. Neurons can be measured from 2D cultures, organoids or other 3D cultures, and slices.
Can I mix neural and glioma cells on the Maestro platform?
Yes. Modeling complex neural-glial interactions using co-cultures or tri-cultures is easy with the Maestro and provides an ideal approach for capturing disease phenotypes in vitro, understanding the effects of tumor metabolites or network activity, and discovering new therapeutic targets. Learn more about neural co-culture and glial interaction.
What kind of metrics can you get from neural activity?
MEA measures from multiple areas of a culture over time, allowing you to go beyond just the firing of individual neurons and evaluate dynamic network activity and the development of functional phenotypes. Learn more about what you can do with our Neural Module.
Can I measure 3D cultures like organoids and spheroids?
Yes. Three-dimensional organoid and cancer spheroid models work on the same plates as 2D cultures. Download our culture protocol, Cancer Spheroids for the Maestro Z, for step-by-step instructions detailing the generation of 3D tumor spheroids for in vitro testing with Axion's label-free cell growth and killing assay platforms.
Can I measure neural activity and viability at the same time?
If you're modeling development of disease progression or screening novel therapeutics, measuring neural activity alone doesn't tell the whole story. The Maestro platform allows you to measure neural activity and viability together with one assay - providing a more complete picture and allowing you to easily characterize the structural and functional integrity of your cells. Learn more about our next-generation technology.



