Prepare the MEA plate and Matrigel solution
- Prepare MEA plate according to standard organoid protocol (PEI-coated surface with standard washing and overnight drying steps)
- Add laminin to final concentration (10 µg/mL) into media containing organoids.
- Prepare enough 100% Matrigel to add at least 30 µL to each well of the MEA plate that will contain an organoid.
Collect the organoids
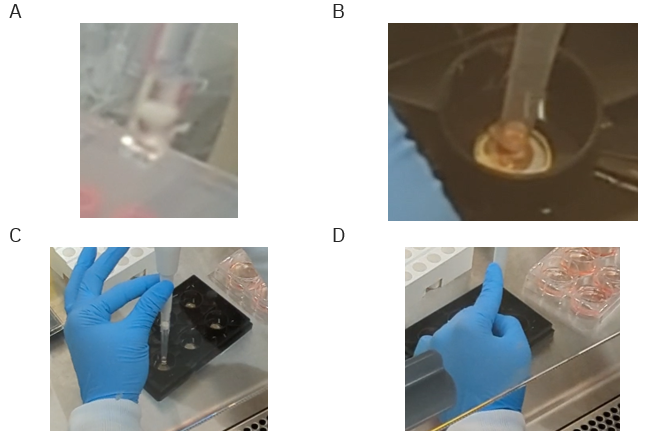
- Using a wide-bore pipette tip or a pipette tip that has been cut to a wide-bore size, gently aspirate some media from the organoid prep wells, and then remove one organoid via pipetting (Figure 1).

Deposit the organoids
- Hold the pipette vertically, allowing the organoid to fall to the bottom of the tip.
- Place the organoid in the center of a well in the MEA plate by holding the pipette vertically.
Tip: Use your other hand to stabilize the pipette tip during the dispense. See Figure 2C & D for examples of stabilizing techniques. - Create scratches using a p200 pipette tip. Place the pipette tip next to the wall of the well such that it is exactly perpendicular to the well bottom. Drag the tip to the other side of the well while applying gentle pressure.
- The MEA plate should be flat on the surface during this transfer step. Repeat steps 4-6 until all wells in the plate have an organoid placed.
Position the organoids
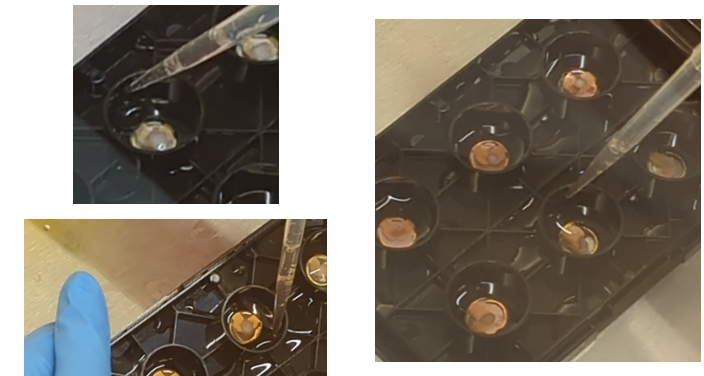
Using the pipette of choice (10 µL or 200 µL), gently aspirate media from the region surrounding each organoid, using that suction to reposition the organoid in the well (e.g., if the organoid is placed too far to the right use suction from the left to reposition the organoid toward the center). See Figure 3 for positioning examples.
Note: The organoids will be sitting in only a small volume of media, but this should be sufficient to maintain hydration for the duration of the plating.
Figure 3. Organoid positioning within MEA plate. - Once the Matrigel is at the appropriate temperature, use a 200 µL pipette tip to aspirate 30 µL. Hold the pipette vertically directly over the organoid and drip the Matrigel onto the organoid (Figure 4).
Note: 30 µL is optimal for 2-3 mm organoids, but this volume can be adjusted based on size.

Incubate the organoids
- Once Matrigel has been added on top of all plated organoids, close the plate lid and transfer to a 37°C/5% CO2 incubator for at least 2 hours, allowing the Matrigel to solidify.
- After 2 hours, transfer the plate back to the biosafety cabinet, and add half volumes of media to each side of each well (Figure 5). If this is a 6-well plate, use 500 µL on either side, paying careful attention to add the media to the wall of the conical feature in the well, allowing the media to gently flow into the well and not disturb the organoids. The plate may be tilted at this stage to assist in media addition.
Troubleshooting detachment
If an organoid becomes detached, which can occur, simply aspirate the media and repeat from Step 8. Consider incubation with Matrigel for longer OR using a larger volume of Matrigel if the organoid is larger.
Maintenance
Once the wells on the plate have media added and organoids are secure, you may proceed with maintaining the culture by performing half media changes regularly. Record from the plate via the Maestro periodically to observe culture maturation over time. You may be able to observe signals within hours of plating the organoids on the MEA, however, this experience may very based on the maturity of the organoids, the differentiation protocol used, and, in general, time on the MEA.
Recording activity
While some organoids may show activity shortly after plating, organoids typically require 1-2 weeks of culture on the MEA plates before showing robust activity. Begin monitoring after 24 hours to identify the optimal analysis window when peak activity and stability is observed.



