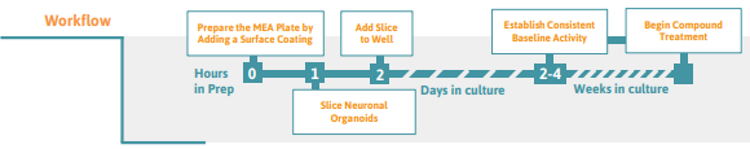
Preparing the multielectrode array (MEA) Plate
-
Coat the entire well surface with a preferred substrate coating (e.g., poly-D-lysine, polyornithine, or 0.1% polyethylenimine).
-
Rinse the surface coating from the well with 200 µl of PBS 4 times, then allow the MEA plate to air dry for at least 2 hours.
Slicing Neuronal Organoids ( >2 mm in diameter)
-
Transfer organoid(s) to a 35 x 10mm petri dish and remove as much of the solution as possible via pipetting and dabbing up with a kimwipe. Carefully pour chilled 4% agarose over organoids and allow to set. Use a metal spatula and pry the agarose block out of the petri dish. Using a razor blade, leave extra space around the organoids for specific trimming and mounting in the vibratome.
Tip: Slowly heat 100 mL 1X PBS (beaker) on a heating stir plate set at 50°C and stir vigorously. Very slowly add 4 g of low melting agarose powder to heated PBS and allow the solution to stir with heating till begins to thicken. -
Mount trimmed block to mounting disk (supplied with vibratome) with super glue and allow the glue to set for at least 3 minutes.
-
Place mounting disk at vibratome and fill the vibratome chamber with ice-cold oxygenated ( 95% O2 and 5% CO2 ) ACSF solution.
-
Slice the neuronal organoids into ~250-400 µm slices. Use high blade frequency and a slow speed.
Tip: Orient the block such that the samplecontaining end will encounter the blade first. If increased stability is necessary due to detachment during slicing, mount another block of 4% agarose behind the initial block containing the organoid(s).
Positioning the Slice on the MEA Plate
-
To transfer the slice to the well, use either a 1mL micropipettor or a transfer pipette. The tip of the pipette of choice should have wide enough bore size to aspirate the slice without damaging it, and this may require cutting the tip end off. The well to transfer to should contain enough warm ACSF solution volume to allow free movement of the slice into position on the electrode array.
Tip: Slices tend to reduce in thickness after some time in culture. -
Using a fine-to-medium tip soft sample brush (e.g., histology camel hair brush) gently position the slice into the desired general orientation over the array and slowly remove the ACSF down to approximately 100-200 µL (enough volume to move the slice, but keep it contained over and touching the array).
-
Use the 1 mL micropipettor with an unmodified tip to gently remove the remaining ACSF, pushing the slice down onto the array via surface tension. It may be necessary to do this in stages – remove a little ACSF and then reposition the slice if it moves during solution removal. Repeat until ACSF is completely removed and slice is adhering to the array – there should be just enough solution (~100 µL) remaining to keep the tissue hydrated.
-
Leave the slice on the array for 20-30 seconds to adhere, repositioning as necessary.
-
Pre-wet the slice grid in ACSF and dab excess with Kimwipe. Place the slice grid on the tissue in one attempt if possible, trying diligently to avoid slice movement. It may be necessary to remove the grid, reposition the slice and try again, remaining conscious to avoid any additional slice movement.
-
Place ~50-200 µL of culture medium on the slice and grid, just enough to keep the tissue hydrated and healthy, and leave the slice in the well for 60 seconds to allow for functional adherence to the array.
-
Look at the final slice placement under the microscope to ensure the position is correct. If the slice is slightly off the target region, very carefully make final placement adjustments, but only if the movement is minute in scale.
-
Gently add 1/2 of the final volume of the medium to each well of the MEA and incubate in a cell culture incubator at 37°C, 5% CO2 . Adding the medium too quickly will dislodge the grid. Recommended final well volumes for 6-well plate (1000 µl).
-
Next day, repeat step 8 to reach the final recommended volume of medium.
-
Exchange 50% of the medium every 2-3 days until the slice cultures are to be analyzed and/or proceed with preferred treatment. Stable baseline activity is usually observed 2-4 days after attachment to the electrodes, but depends on the maturation stage of the initial neuronal organoid.


